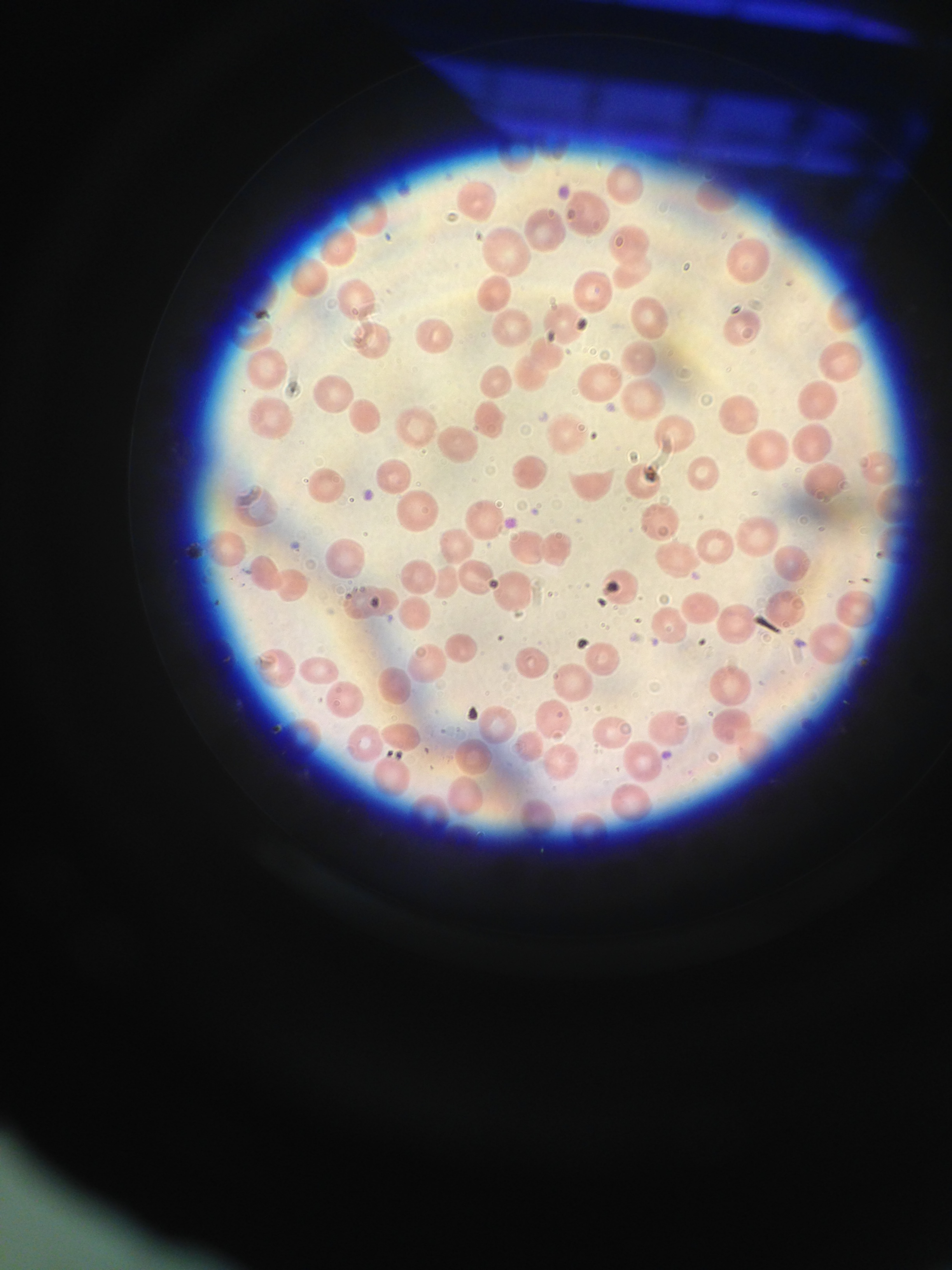
Case Presentation: Our patient is a 60 year-old woman with a history of metastatic adenocarcinoma of unknown primary who had undergone a total of 26 cycles of gemcitabine chemotherapy alone and in combination with oxaliplatin. She most recently received gemcitabine one month prior to presentation. Four weeks prior to presentation, the patient reported new-onset lower extremity edema and fatigue to her oncologist. At that visit, she was found to be anemic (hemoglobin of 7 g/dL from a baseline of 9 g/dL) and had new rise in her creatinine to 1.3 mg/dL from a baseline of 1 mg/dL. She was transfused four units of packed red blood cells over the next week for symptomatic anemia. Two weeks prior to presentation at a follow-up visit, she was noted to have new hypertension to 170/80, worsening lower extremity edema, worsening renal insufficiency (creatinine of 1.4 mg/dL) and large blood and large protein in her urine. One week later, she reported persistent fatigue and shortness of breath. Her labs revealed persistent anemia and worsening renal insufficiency (creatinine 1.8). She was subsequently admitted to the hospital for transfusion and diagnostic work-up. Upon admission, LDH was 1643 U/L, haptoglobin was 10 mg/dL and a platelet count was 111 x 109 /L (baseline 300 x 109/L). A peripheral smear showed 2-3 schistocytes per HPF. Her clinical picture and lab work appeared consistent with gemcitabine-induced TMA. She was treated with antihypertensive medications and transfusions. After discussion with her oncologist, gemcitabine was discontinued and with supportive therapy, her renal function and cell counts normalized.
Discussion: Thrombotic microangiopathy (TMA) is an umbrella term that encompasses a diverse group of hereditary and acquired syndromes characterized by microangiopathic hemolytic anemia, thrombocytopenia and organ injury. Among acquired causes, drug-mediated TMA is relatively common. Recently, the chemotherapeutic agent gemcitabine has increasingly been implicated in acquired TMA cases. It has been reported that TMA complicates gemcitabine chemotherapy in anywhere from 0.15 to 0.3% of cases. As gemcitabine is commonly used in several chemotherapy regimens it is crucial that oncologists and hospitalists be cognizant of this rare but potentially fatal toxicity.
Conclusions: Gemcitabine is a potent chemotherapeutic drug used in treating several cancers including breast, lung and pancreatic cancer. Common side effects of gemcitabine include nausea and vomiting, however, thrombotic microangiopathic complications have been increasingly reported. Clinicians who care for patients receiving this chemotherapy agent must remain astute about the possibility of this rare but potentially fatal complication.