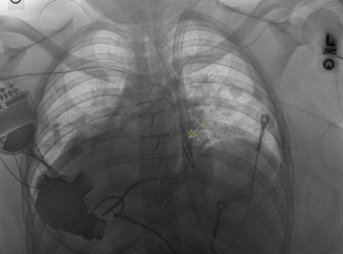
Case Presentation: The patient is a 24-year-old female with Situs Inversus Totalis (SIT), obesity, Stage D non-ischemic dilated cardiomyopathy (NIDCM), history of out of hospital cardiac arrest (ventricular fibrillation arrest) with subsequent dual chamber implantable cardioverter-defibrillator (ICD) placement who was managed as an outpatient on home inotrope therapy as a bridge pending left ventricular assist device (LVAD) placement. She underwent LVAD placement with a HeartMate III via full sternotomy approach. Following surgery, she was extubated on post operative day 1 (POD 1), utilizing inotropic support via Dobutamine and Epinephrine with intermittent hypertensive episodes necessitating Nicardipine. While initially stable, her respiratory status began to decline despite medical therapy via Epoprostenol, diuresis, and inotropes. On POD 2, her arterial PCO₂ levels were elevating with further worsening of her respiratory status, needing bilevel positive airway pressure (BiPAP). Concurrently, she exhibited signs of right ventricular (RV) dysfunction such as increased central venous pressure (CVP) and decreased cardiac index (CI), alongside acute kidney injury (AKI) with decreased urine output.Due to the worsening RV failure and deteriorating respiratory status, patient underwent placement of a 29F ProtekDuo cannula, inserted via the left internal jugular vein (IJV), establishing veno-pulmonary artery extracorporeal membrane oxygenation (V-PA ECMO) for RV support via percutaneous right ventricular assist device (pRVAD) along with reintubation. Her condition improved with increased urinary output along with extubation on POD 4. The patient’s RV function and respiratory status continued to improve and on POD 6, the ProtekDuo cannula was removed. The patient remained stable with signs of recovery from AKI and RV dysfunction, weaning to mono-inotropic support pending transplant candidacy.
Discussion: The utilization of LVADs has become standard therapy for end-stage heart failure, as a bridge to transplantation or destination therapy. In patients with SIT, the anatomical variations require modifications in cannulation sites and device positioning. In this case, the implantation of the LVAD was achieved by adapting the surgical technique to accommodate the reversed anatomy. The surgeon elected to perform midline sternotomy given the preference for direct visualization of the atypical anatomy of this patient with SIT. The LVAD was connected upside-down to the inflow conduit with the outflow cannula placed around the heart and anastomosed to the aorta.Right ventricular failure (RVF) is a known complication following LVAD implantation, occurring in 20-40% of cases. The hypothesized mechanism of RVF includes volume overloading of the right ventricular due to an increased flow in systemic circulation or an interventricular septal shift into the LV compromising RV contraction. As a result, temporary mechanical cardio-circulatory support may be needed. The Protek Duo dual-lumen cannula offers a minimally invasive option for temporary RV support (pRVAD). Its mode of insertion reduces surgical trauma and can be particularly advantageous in patients with complex anatomies.
Conclusions: The management of cardiac failure in patients with SIT requires consideration of anatomical differences and this case demonstrates that with appropriate adaptations, advanced mechanical circulatory support devices like LVADs and pRVAD cannulas can be utilized.