Background: Intravenous vancomycin treatment is often necessary beyond hospitalization. Peripherally inserted central catheters (PICCs) are usually placed for this indication. Whether the less invasive midline catheters (midlines) are safe alternatives for outpatient parenteral antimicrobial therapy (OPAT) with vancomycin is unclear. We compared the safety of midlines and PICCs among patients receiving vancomycin for OPAT.
Methods: From January 2017 to November 2023, trained abstractors at 69 hospitals participating in the Michigan Hospital Medicine Safety Consortium (HMS) collected patient level information on midline and PICC placements on patients admitted to general medical units who required OPAT. Primary outcome was any major device complication, such as catheter-related bloodstream infection (CRBSI) or catheter-related venous thromboembolism (CR-VTE). Secondary outcome was any minor device complication, such as catheter dislodgement, occlusion, tip migration, infiltration, superficial thrombosis, or exit site problems (including leaking and infection), and device failure, defined as premature device removal due to any device complication. We assessed the association between device type (midlines versus PICCs) and outcomes among patients receiving vancomycin for OPAT. All analyses were performed using cox proportional hazards regression to account for variation in dwell and other prespecified covariates.
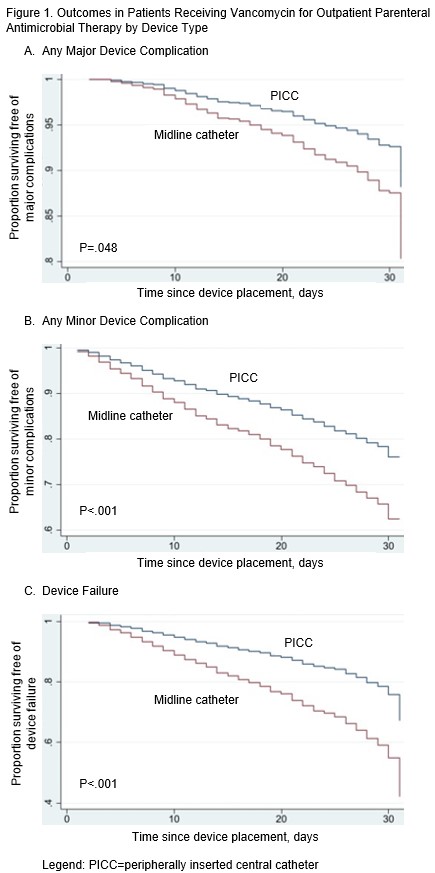
Results: Of 2,387 devices used to administer vancomycin for OPAT, 429 (18.0%) were midlines and 1,958 (82.0%) were PICCs. Median dwell was 13 days (Interquartile Range [IQR], 8-18.5 days) for midlines and 21 days (IQR, 13-30 days) for PICCs, p<.001. Median OPAT days was 12 days (IQR, 8-19 days) for midlines and 20 days (12-29 days) for PICCs, p<.001. A major device complication occurred in 4.9% (118/2,387) of all vancomycin recipients, including 4.9% (21/429) of those with midlines and in 5.0% (97/1,958) of those with PICCs, p=.96. CRBSI was observed in 2.8% of midlines and 3.3% of PICCs, p=.58; while CR-VTE was seen in 2.1% of patients with midlines and 1.7% of those with PICCs, p=.61. After adjusting for variation in dwell and other covariates, infusing vancomycin through midlines versus PICCs was associated with higher risks of major complications (adjusted Hazard Ratio [aHR], 1.70; 95%CI, 1.01-2.87), and device failure (aHR, 2.17; 95%CI, 1.65-2.86). The risk of minor device complications was also higher with midlines (aHR, 1.73; 95%CI, 1.31-2.27), especially exit site problems (aHR, 15.85; 95%CI, 8.23-30.56).
Conclusions: When used for outpatient parenteral antimicrobial therapy with vancomycin, midlines are associated with higher risks of serious device-related adverse events and device failure compared to PICCs.