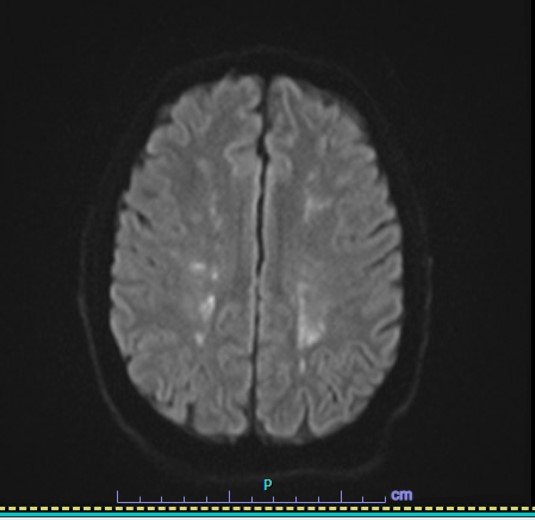
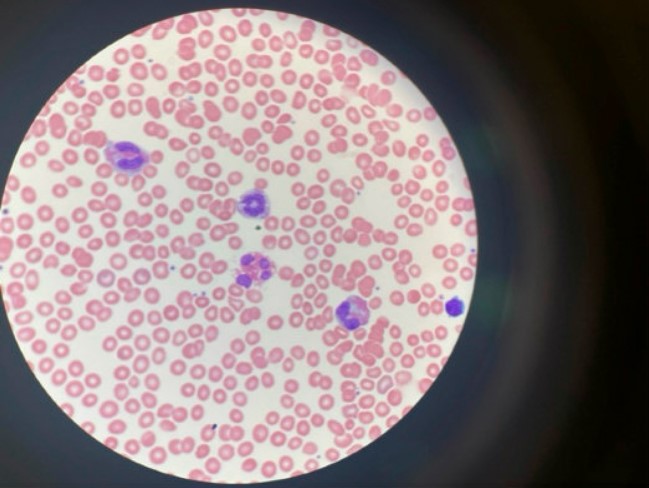
Case Presentation: A 38-year-old Caucasian male with no significant prior medical history presented with an acute CVA based on the presence of fatigue, blurry vision, ataxia, headache, and numbness. His MRI showed “multiple small foci of high DWI/ADC signal” in the bilateral cerebral and cerebellar hemispheres consistent with small acute infarcts. Unremarkable CTA brain/neck, ECHO, and TEE ruled out a cardiac etiology. A CBC with differential showed eosinophil levels near 41% and absolute eosinophil counts of 9.05 x10^9/L. The patient was started on methylprednisolone. He became increasingly encephalopathic with periods of aggression and confusion. His EEG showed generalized slowing. A consult with ID was inconclusive and the patient was treated with Solumedrol, Hydroxyurea, Bactrim, Valtrex, and Ivermectin to cover an infectious etiology as his CBC continued to show increasing eosinophil levels. A bone marrow biopsy showed hypereosinophilia and a FIP1L1-PDGFRA rearrangement but no evidence of lymphoma or acute leukemia. FISH identified a deletion of the CHIC2 gene on chromosome 4q12 resulting in the fusion of FIP1L1-PDGFRA, which resulted in steroid-refractory hypereosinophilia. Once Imatinib was started, eosinophil levels dropped from 13,000 μl to 700 μl and WBC counts dropped from 45 × 10^9 /L to 4.8× 10^9 /L within a few days. He began experiencing significant physical and cognitive improvements. A cardiac MRI showed no signs of eosinophilic myocarditis. He remains well with outpatient management with rehab and hematology-oncology and continued Imatinib and Eliquis.
Discussion: Primary Hypereosinophilic Syndrome (HES) involves underlying stem cells, myeloid, or eosinophilic neoplasm and is defined by a marked blood absolute eosinophil count (>1.5 x 10^9 /L). It is a diagnosis of exclusion once other causes of hypereosinophilia (allergies, parasites, malignancies) have been ruled out. The estimated prevalence of HES is between 0.36 to 6.3 per 100,000. Among the identifiable causes of HES, the FIP1L1-PDGFRA fusion gene is the most common and affects between 10-14% of patients presenting with HES. These patients classically do not respond to corticosteroid therapy and typically have an aggressive course with poor prognosis without proper treatment with Imatinib. The response to Imatinib is rapid with the majority of patients experiencing clinical and hematological resolution of abnormalities within the first month. Patients with HES are at high risk for developing thrombosis. A cohort study involving 71 adults with HES showed that thrombotic events were common with 24% of the study population experiencing at least 1 event. The presence of a molecular aberration on next-generation sequencing, such as FIP1L1-PDGFRA, was associated with a 5-fold increase in the risk of thrombosis. Although this disease is rare, prompt diagnosis and treatment lead to a better prognosis. In a retrospective study analyzing 43 patients, the overall survival rate was 80% at 5 years and 42% at 10 and 15 years following initial diagnosis.
Conclusions: Hypereosinophilic syndrome is a rare condition that can lead to fatal outcomes because of the potential for end-organ damage if treatment is delayed. There are a variety of etiologies for this condition including infectious parasites, genetic mutations such as the FIP1L1-PDGFRA variant, underlying malignancy, and even idiopathic causes. A multidisciplinary approach with early hematology intervention is essential to ensure safe patient outcomes.