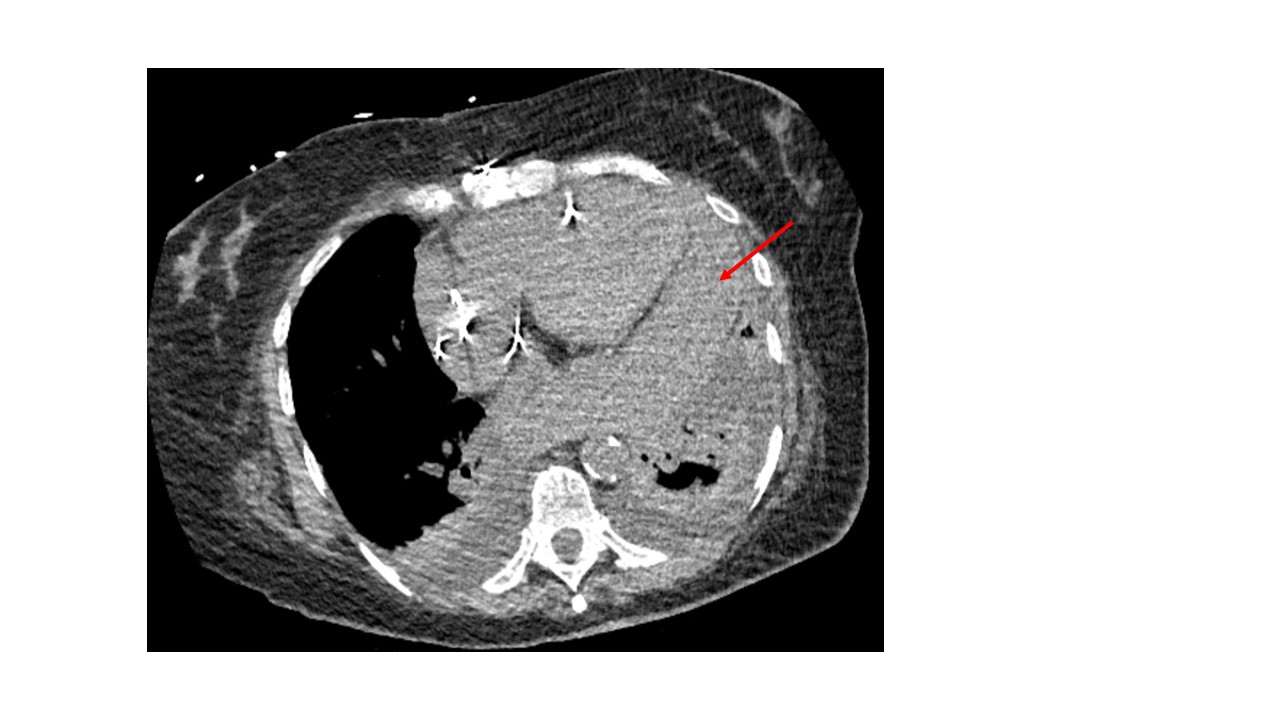
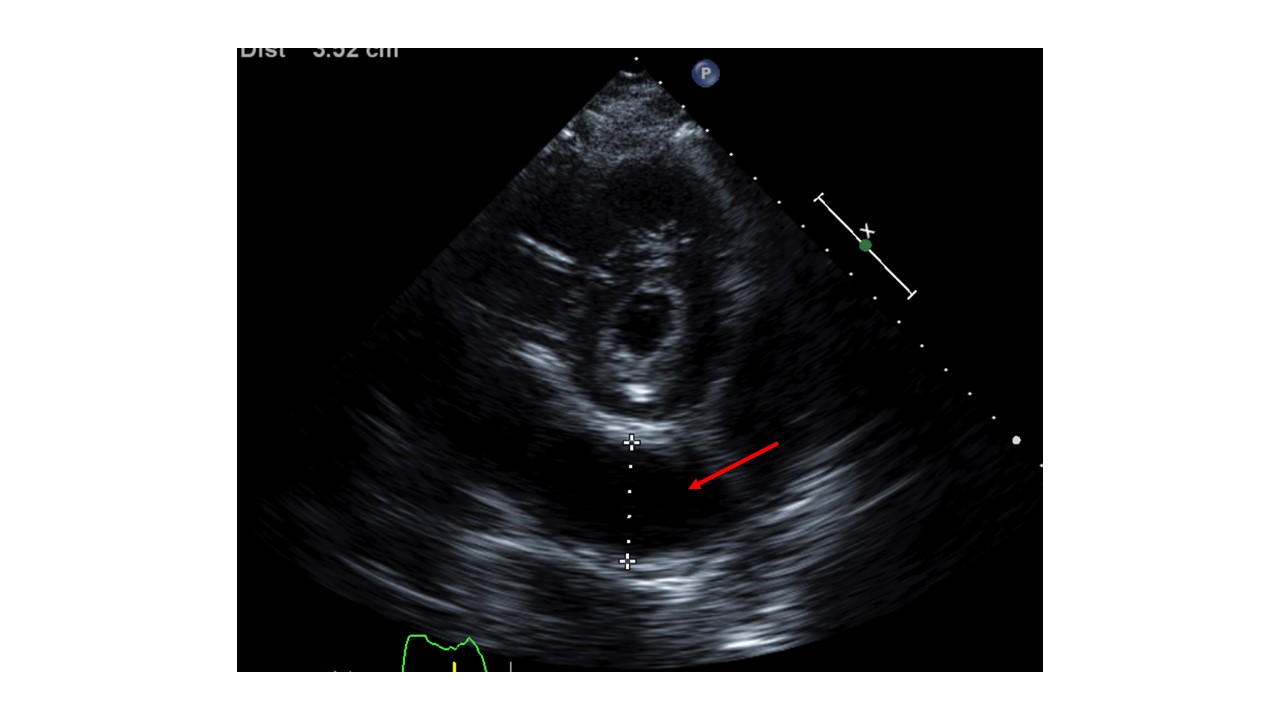
Case Presentation: A 65-year-old female with a history significant for persistent atrial fibrillation, on apixaban, aortic and mitral bioprosthetic valve replacement, dual-chamber permanent pacemaker (PPM) implantation for heart block 2 weeks prior presented with worsening shortness of breath, dry cough, and generalized weakness for 4 days. Initially, she was found to be hemodynamically stable. Labs revealed leukocytosis (15,600/uL; Ref 4000-11,000/uL) and normal troponin levels. Imaging with chest -x ray showed left lower lobe pneumonia and positive Streptococcus pneumoniae urinary antigen. She was treated with antibiotics, but her hospital course was complicated by worsening hypoxia, hypotension, and lactic acidosis. Due to concern for empyema, a CT chest was obtained which revealed loculated hemopericardium. Subsequent transthoracic echocardiogram (TTE) revealed LVEF of 50-55% and circumferential pericardial effusion with tamponade physiology. Electrocardiogram showed no acute ischemic changes. Her apixaban was held and cardiothoracic surgery was consulted for hemorrhagic shock likely secondary from PPM lead ventricular perforation. She underwent limited left thoracotomy, drainage of effusion, and closure of the inferior apical defect. There was no evidence of pacemaker lead coming through the surface of either ventricle intraoperatively. The infectious and inflammatory workup of pericardial fluid was negative, and the cause of hemopericardium was determined to be the micro-perforation of the ventricle from PPM lead which did not warrant lead revision. Repeat TTE showed no re-accumulation of pericardial effusion. The patient recovered and was discharged in stable conditions with outpatient cardiology follow-up.
Discussion: Lead perforation is an uncommon cause of pericardial effusion accounting for 0.1%-0.8% of pacemaker placements. (1) Risk factors include older age, female sex, steroid use, anticoagulant use, and low body mass index. Patients are often asymptomatic but may develop chest pain, dyspnea, syncope, or fatal complications. Chest X-ray and echocardiography can help in visualizing the pericardial effusion and lead displacement, but CT chest is more helpful in delineating the perforation and lead position. The management is based on clinical presentation. Extraction is avoided in patients with chronic asymptomatic lead perforations due to the associated complications. However, urgent surgical management is needed in hemodynamically unstable patients, progressive pericardial effusion with cardiac tamponade or large pleural effusions with respiratory compromise as was the case with our patient. (2)
Conclusions: Cardiac perforation by PPM lead is rare but associated with high morbidity and mortality. Therefore, a high clinical suspicion should be maintained in patients with cardiac devices presenting with chest pain, especially on anticoagulation for timely management.