Background: Racial minorities are underrepresented in cancer-related clinical trials. One commonly recognized contributor to this is medical mistrust.
Methods: This study used data from the 2020 National Cancer Institute’s Health Information National Trends Survey (HINTS), a cross-sectional, nationally representative sample of 3865 participants. We sought to determine the prevalence of medical mistrust and its impact on cancer information seeking behavior and motivations to participate in clinical trials.
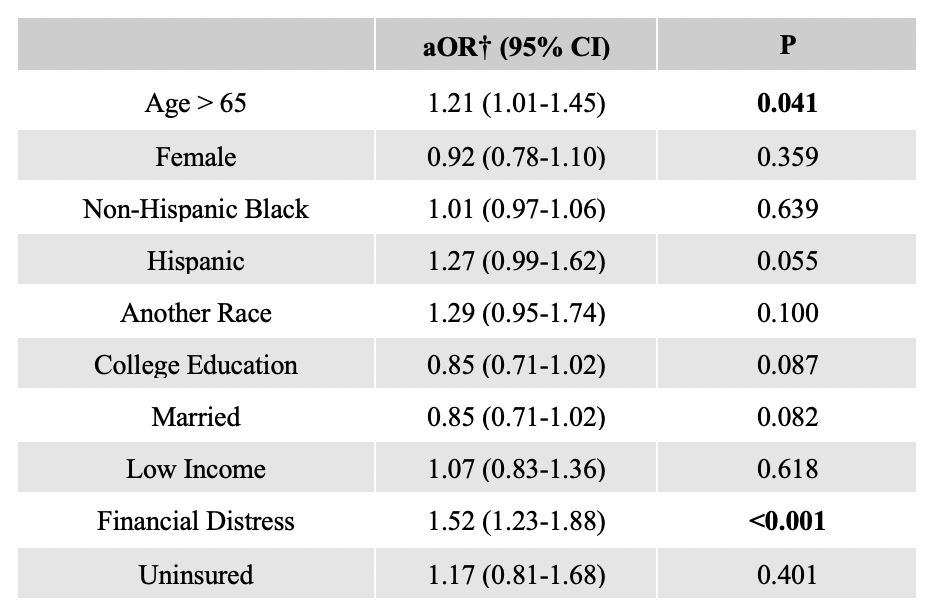
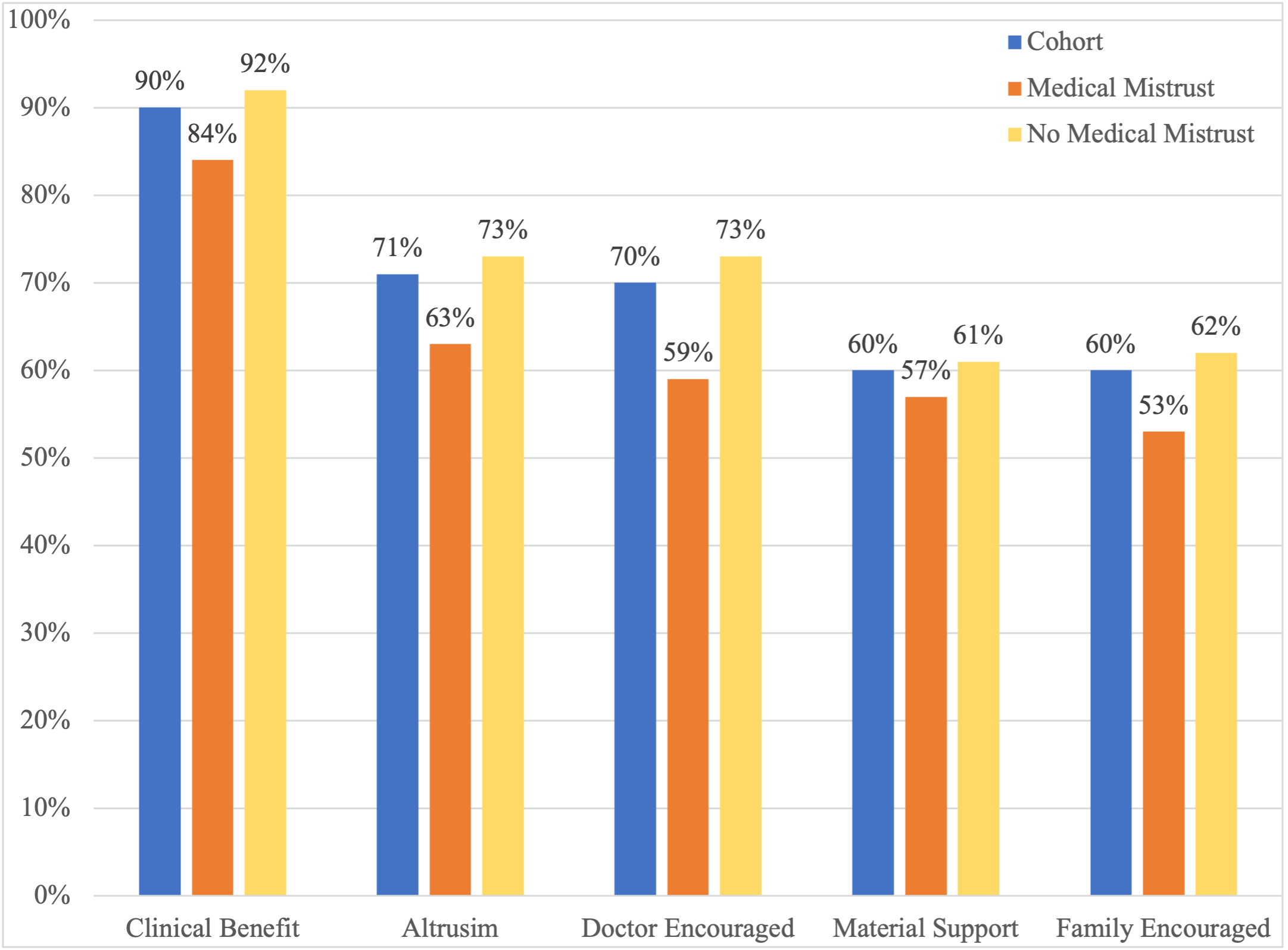
Results: In total, 3452 participants were included in the analysis; 24% were determined to have medical mistrust. In multivariable analysis, race was not independently associated with medical mistrust, but older age and financial distress were. Those with medical mistrust were less likely to seek information about cancer or clinical trials from doctors or health care providers. They were also more likely to report “I don’t know anything about clinical trials” and were less likely to report altruism, or receiving a clinical benefit, material support, and doctor or family/friend encouragement as motivating factors for trial participation. Higher knowledge about clinical trials was associated with higher motivation to participate regardless of medical mistrust.
Conclusions: This study does not support medical mistrust as a mediating factor for the underrepresentation of minorities in clinical trials. People with medical mistrust report differences in information seeking behavior and motivating factors for clinical trial participation. Increasing clinical trial knowledge may increase motivation to participate in people with medical mistrust as well as those without.