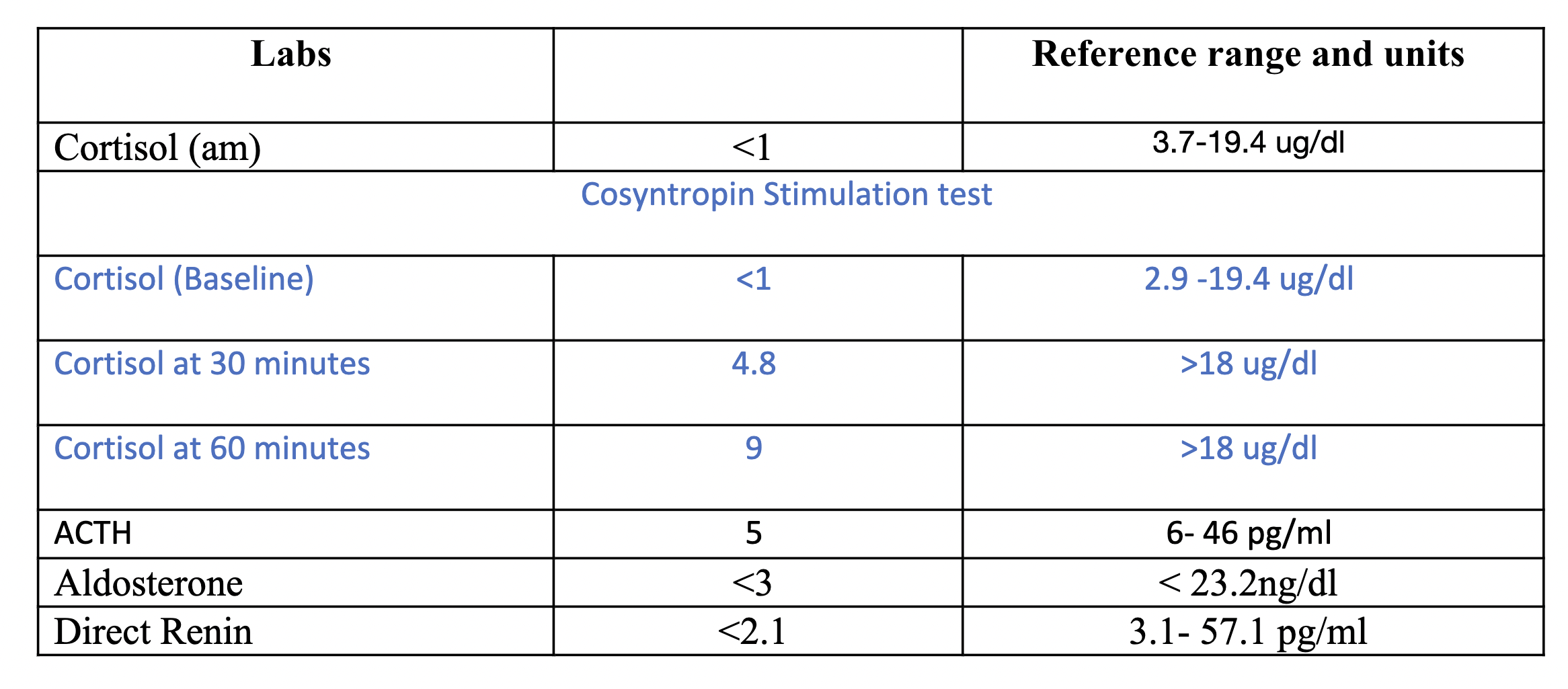
Case Presentation: A 43-year female with history of gastric adenocarcinoma and Krukenberg tumor, a metastatic disease to the ovaries composed of mucin-rich signet-ring cells, status post left salpingo-oophorectomy and chemotherapy with FOLFOX [leucovorin calcium (folinic acid), fluorouracil, and oxaliplatin] and Opdivo (Nivolumab) presented due to symptoms of dysuria and generalized pain. She was febrile and hypotensive. She was started on IV fluids and empiric antibiotics. Initial lab work were unremarkable. Her blood and urine culture remained negative but the patient continued to have frequent episodes of hypotension, weakness, fatigue, nausea and dizziness. The patient had required stress dose steroids to maintain blood pressure. Subsequent workups for possible sources of sepsis were unrevealing. This prompted a check of random morning cortisol level. After remaining steroid-free for 24 hours, her morning cortisol level was < 1 (normal 3.7-19.4 ug/dl). Cosyntropin simulation test showed cortisol level of 4.8 ug/dl at 30 minutes and 9 ug/dl at 60 minutes. (normal >18 ug/dl). ACTH level of 5 (normal 6-46 pg/ml), aldostreone of < 3 (normal < 23.2 ng/dl), direct renin of < 2.1 (normal 3.1-57.1 pg/ml). These lab parameters indicated that the patient had secondary adrenal insufficiency (AI), presumably a secondary etiology such as hypophysitis. Upon further evaluation, it was discovered that patient was started on FOLFOX and Opdivo 2 years ago for her Krukenberg tumor. FOLFOX was discontinued within 3 months, but Opdivo was continued. Her last dose of Opdivo was 1 month prior to this presentation. Her AI was attributed to the side effects of Opdivo. She had symptomatic improvement after being on hydrocortisone 10 mg twice daily and she was discharged with an AI bracelet.
Discussion: Immune checkpoint inhibitors, like Opdivo, are vital in cancer treatment but can cause immune-related adverse events (irAEs) affecting multiple organ systems. One such complication is hypophysitis, inflammation of the pituitary gland, which can lead to ACTH deficiency and secondary AI. Although rare, occurring in about 0.5-2% of patients, this side effect can be life-threatening, particularly with delayed diagnosis. Symptoms like fatigue, dizziness, and loss of appetite are common during cancer treatment and often dismissed but these non-specific signs can mimic other conditions, such as sepsis, leading to unnecessary treatments like antibiotics. Opdivo-induced AI, though uncommon, should always be considered, as early detection is crucial for preventing severe outcomes. In our case, recurrent hypotension initially prompted an infectious workup, but secondary AI was ultimately diagnosed in time. While there are no guaranteed preventive measures for Opdivo-induced secondary AI, strategies for early detection, such as measuring baseline ACTH levels before starting treatment are important. Additionally, studies suggest that recovery of the hypothalamic-pituitary-adrenal axis may occur, so regular monitoring over time could be beneficial to assess recovery.
Conclusions: In conclusion, it is crucial to recognize the potential risks of immune checkpoint inhibitors and implement careful monitoring strategies. Secondary AI can be effectively managed with early diagnosis through a high suspicion index. Clinicians’ awareness of such rare side effects is essential for ensuring patient well-being through timely recognition and intervention.