Case Presentation:
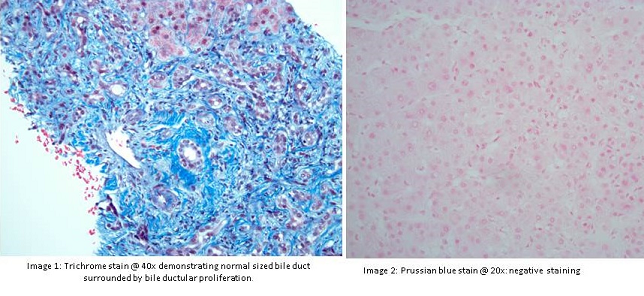
A 69 year old female presented with elevated alkaline phosphatase (1509 U/L), total bilirubin (2.4 mg/dl), alanine transaminase (44 U/L), aspartate transaminase (107 U/L). Her liver function tests had been normal prior to this including alkaline phosphatase which was also in normal range but was progressively increasing in the past 10 years. Her serology over the years was negative and imaging including ultrasound abdomen was normal. Magnetic resonance cholangiopanreatography (MRCP) showed no intrahepatic ductal dilation, normal caliber common bile duct and a distended gallbladder with dependent sludge and small gallstones; diffuse heterogenous enhancement of liver concerning for early cirrhosis, and new volume ascites. Abdominal exam was benign and it was felt that the fluid in the abdomen was related to her congestive heart failure and that some of her fluctuating enzymes might have been related to right-sided failure. Her autoimmune serology including anti-neutrophil antibody and anti-mitochondrial antibody were negative. Her viral markers, metabolic markers, and other autoimmune markers were negative. Transjugular liver biopsy showed findings consistent with chronic cholestatic jaundice with bridging ductular reaction, findings which were worrisome for chronic extrahepatic biliary obstruction, but MRCP was relatively normal. Upward trend in alkaline phosphatase was seen after lisinopril was started. ACE inhibitor may rarely cause cholestatic pattern of liver injury which closely mimics biliary obstruction. Alkaline phosphatase started to trend down when lisinopril was held at 5 months follow up.
Discussion:
Lisinopril, a FDA approved angiotensin converting enzyme inhibitor (ACEi) is widely prescribed for heart failure, hypertension and microalbuminuria. Dermatological, renal and hematological side effects of ACEi are well recognized. ACEI induced cholestatic hepatotoxicity has been reported as occurring from 2 weeks to 4 years after initiation. Recovery is possible but is usually delayed (as long as 6 months) and is dependent on the etiology. A rare case of fulminant hepatic failure has been reported with lisinopril use. Here, we report a case of lisinopril induced cholestatic hepatitis culminating into sub-acute liver failure.
Conclusions:
ACE inhibitors are widely used in heart failure and hypertensive patients. Hepatotoxicity with ACEi, usually cholestatic in nature, is reported in this patient. Mechanism of injury includes mainly idiopathic hypersensitivity reaction. Physicians must be aware of this rare side effect of lisinopril. Currently there are no guidelines to monitor liver enzymes following initiation of ACEi, more research is required to delineate guidelines to prevent hepatotoxicity in such patients.