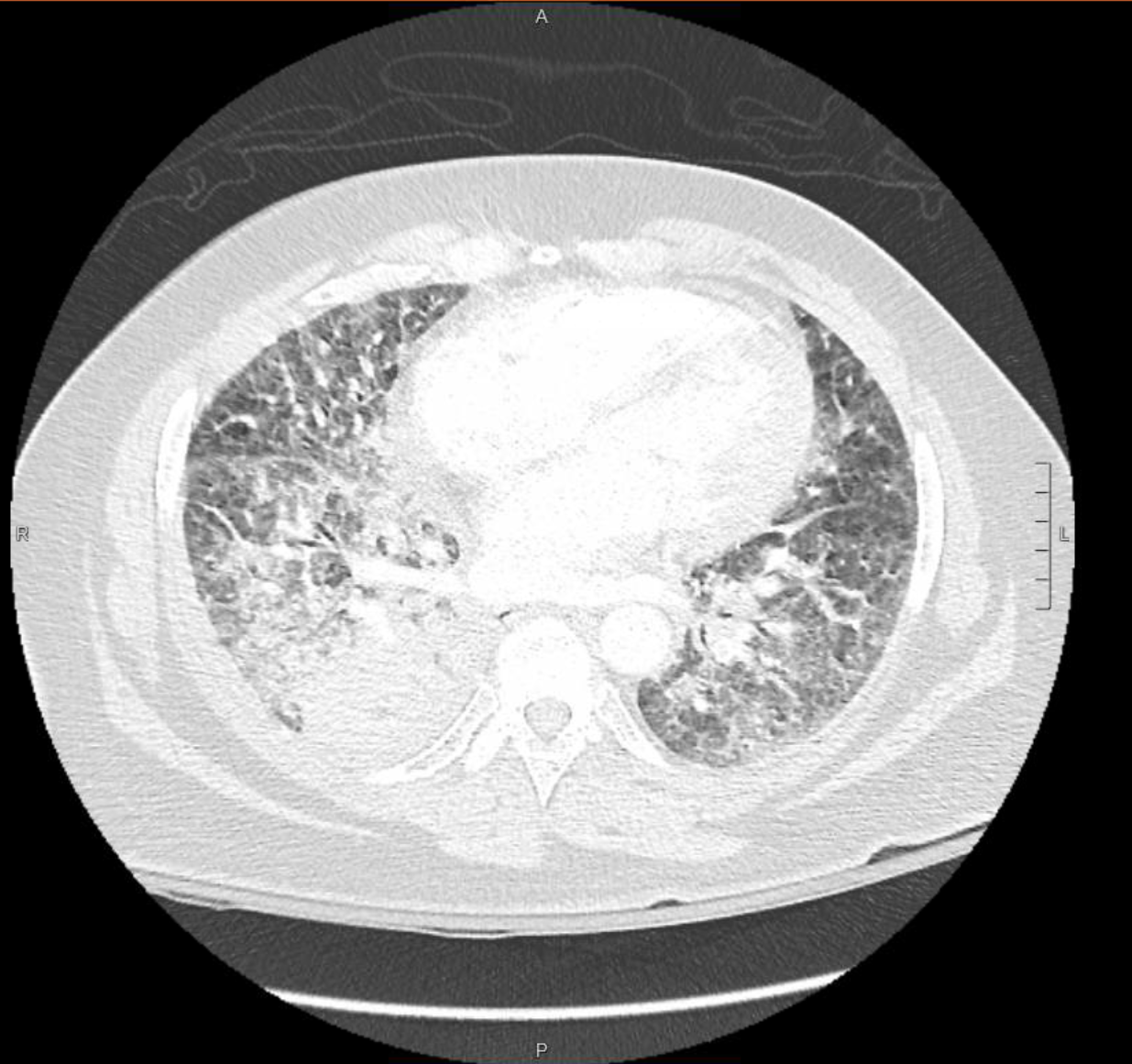
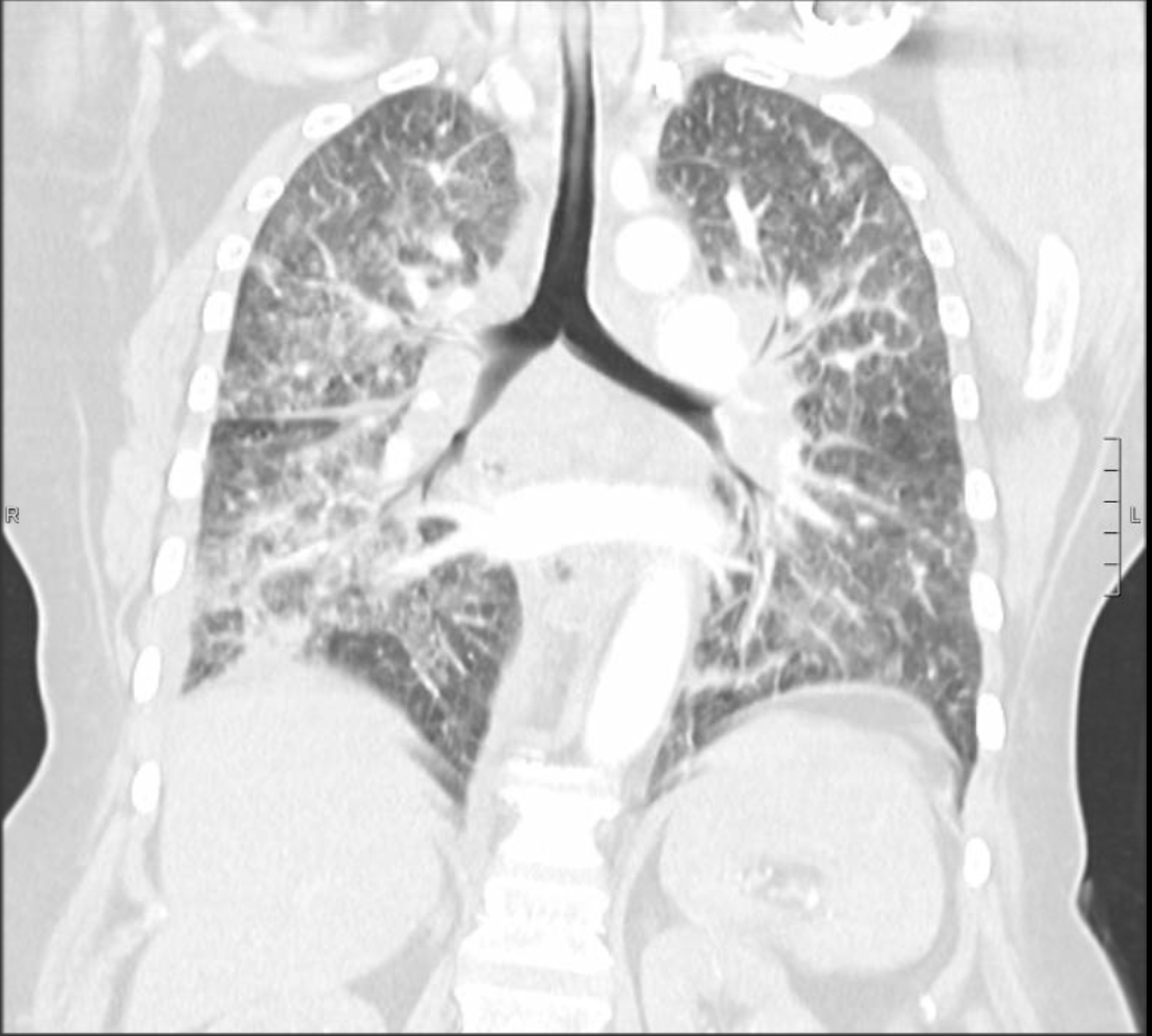
Case Presentation: A 59-year-old female with a history of Asthma, COPD and tobacco use, unknown to have malignancy, presented to the emergency department complaining of dyspnea, cough and back pain. She had tachycardia, tachypnea and oxygen saturation was 91% on 2 liters oxygen. Physical exam was significant for bilateral wheezing.Initial workup showed a high white count of 12.1 K/uL (normal range 4 to 11). Electrolytes and renal function were within normal ranges. Troponin and BNP were normal. Chest radiograph showed diffuse interstitial and airspace opacities. CT angiography of chest showed bilateral filling defect in pulmonary arteries, intralobular septal thickening with ground glass appearance in the right lower lobe and bulky mediastinal and hilar lymphadenopathy. Echocardiogram showed elevated pulmonary artery pressure. She was started on heparin, breathing treatments, antibiotics, intravenous steroids and diuretics. Patient had increasing oxygen requirements throughout admission requiring ICU transfer and invasive ventilation. She had cardiac arrest while being placed on extracorporeal membrane oxygenation for severe respiratory failure and lactic acidosis. Autopsy showed lung adenocarcinoma with extensive lymphangitic spread and metastases to hilar and mediastinal lymph nodes, liver, kidneys and adrenals.
Discussion: Lymphangitic carcinomatosis was first described in 1829. It is a rare condition in which cancer cells spread to the lymphatic system. Infiltration can involve any lymphatic vessels, but most commonly occurs in the pulmonary lymphatics where it is called Pulmonary Lymphangitic Carcinomatosis (PLC). The most common primary tumors associated with PLC are lung, breast, stomach, pancreas and prostate cancer. 80 % of PLC is caused by adenocarcinomas. The mechanism of lymphatic spread remains unclear. Hematogenous spread of malignant cells to lymphatic system is a suggested theory. Another suggestion is retrograde spread from mediastinal and hilar lymph nodes into pulmonary lymphatic system. The mean age of PLC occurrence is 49.21 years with no difference between sexes. Most patients with PLC have a known history of cancer. In some cases, PLC is the first presentation of an occult malignancy. Patients present with nonspecific findings on history and physical exam. Common symptoms include progressive dyspnea, cough, weight loss and fever. Imaging studies are not accurate in diagnosing PLC, but they can help rule out other causes of respiratory symptoms. CT scan of chest can demonstrate bronchovascular bundle thickening, smooth or nodular thickening of interlobular septa, either focally or widespread with ground glass opacities. Studies have shown that hybrid PET/CT scan with FDG tracer has 86% sensitivity and 100% specificity in the detection of PLC. Definitive diagnosis is by transbronchial or surgical lung biopsy, which can only be done on stable patients. Most often, PLC is diagnosed retrospectively on autopsy evaluation. PLC represents an advanced stage of malignancy with life expectancy about 6 months.
Conclusions: Pulmonary Lymphangitic Carcinomatosis is an uncommon condition, defined as the infiltration of cancerous cells into lymphatic vessels. It is mostly associated with adenocarcinoma of breast, lung, stomach, pancreas and prostate. Diagnosis is challenging and typically occurs postmortem. PLC represents advanced malignant stage with poor prognosis.