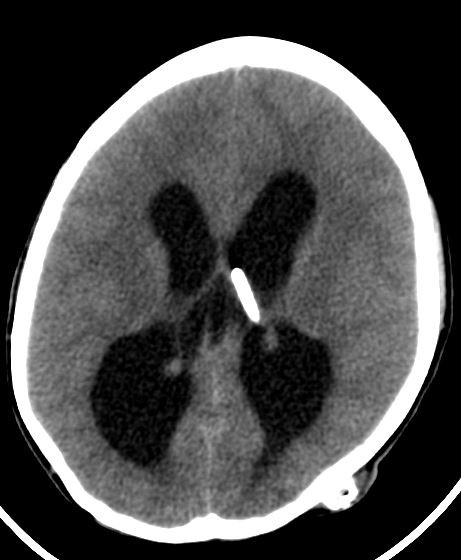
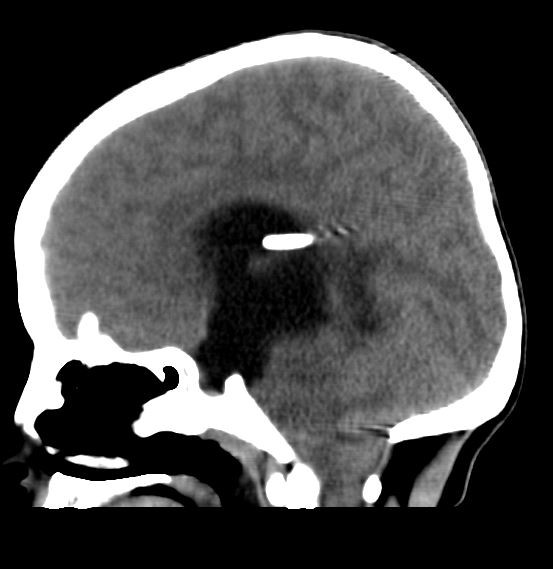
Case Presentation: A 14-year-old female with a history of hydrocephalus status-post ventriculoatrial (VA) shunt placement five years prior presented for acute lethargy characterized by difficulty waking her from sleep. Vital signs were T 36.6 C, pulse 72, respirations 20, BP 148/97, and SpO2 100% with exam notable for lethargy, spontaneous eye and limb movement, pupils 3 mm and reactive, and heart with regular rate and rhythm without murmur. A broad evaluation occurred. Within 1 minute after urinary catheterization, she became apneic. A pulse was present, oxygen saturations fell, and bag-mask ventilation took place followed by intubation. Head CT revealed obstructive hydrocephalus (enlargement of the lateral and third ventricles and tonsillar and brainstem herniation). She was taken to the operating room where her VA shunt was replaced; however, the distal catheter of the original shunt was found to be disconnected and could not be removed. A post-operative echocardiogram was obtained to localize the catheter, which incidentally showed severe biventricular systolic dysfunction with an ejection fraction (EF) of 25%, dilated pulmonary artery, mildly dilated aortic root, mildly dilated left coronary artery, and patent foramen ovale. Post-operative brain MRI demonstrated decompressed ventricles with no evidence of infection (no meningeal enhancement). Electrocardiogram (EKG) revealed sinus tachycardia and flat T waves. Therapies included milrinone, enoxaparin, aspirin, and broad-spectrum antibiotics. Serum tests were remarkable for white blood cell count 23,000, brain natriuretic peptide 16,517 ng/L, troponin 609 ng/L, and normal CRP and ESR. Electroencephalogram revealed focal epileptiform anomalies, but no seizures were observed. CSF studies were deferred due to risk of introducing infection after shunt replacement. Nasopharyngeal polymerase chain reaction was positive for rhinovirus/enterovirus. Pseudomonas grew on urine culture, and she received appropriate antibiotics. Whole exome sequencing showed a USPX mutation, associated with a dilated aortic root but not acute heart failure. Echo on hospital day 3 showed EF 50-55%. She recovered to her neurologic baseline. A diagnosis of neurogenic stunned myocardium was made.
Discussion: The differential for her acute heart failure included myocarditis, endocarditis, myocardial infarction, coronary ischemia, genetic, and sepsis. The cause of her shunt malfunction was not known. Due to rapid recovery of heart function after correction of her acute brain herniation with otherwise non-specific test results, a diagnosis of neurogenic stunned myocardium was made. Neurogenic stunned myocardium lacks formal diagnostic criteria but is considered a diagnosis of exclusion that includes regional heart wall dysfunction, elevated cardiac biomarkers, and non-specific EKG findings. Similarly, pathophysiology is unknown, with postulation that heart failure results from sympathetic nervous system stimulation, myocardial microvascular dysfunction, or genetic predisposition following an acute brain injury. Although there are three prior reports of neurogenic stunned myocardium occurring in children in the setting of hydrocephalus, this is, to our knowledge, the first report of a case occurring secondary to a shunt malfunction in a pediatric patient.
Conclusions: This is a case of neurogenic stunned myocardium – a rare, but probably underdiagnosed, phenomenon – occurring secondary to shunt malfunction.