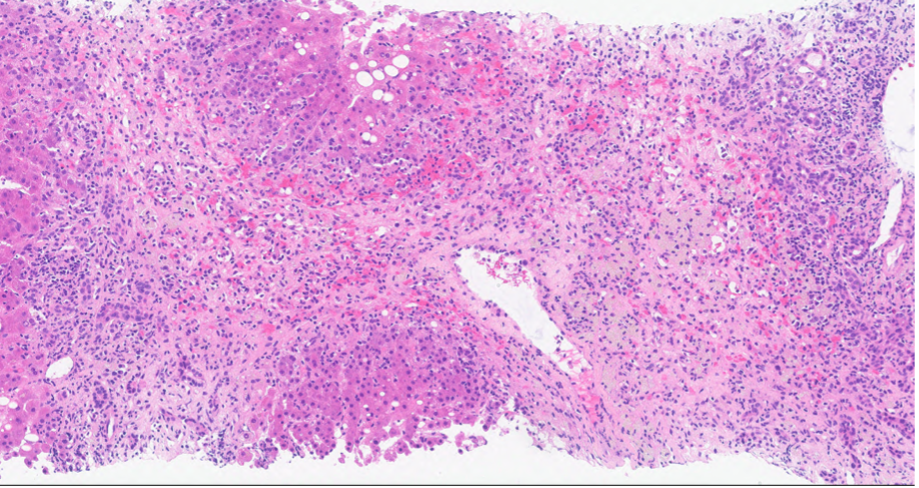
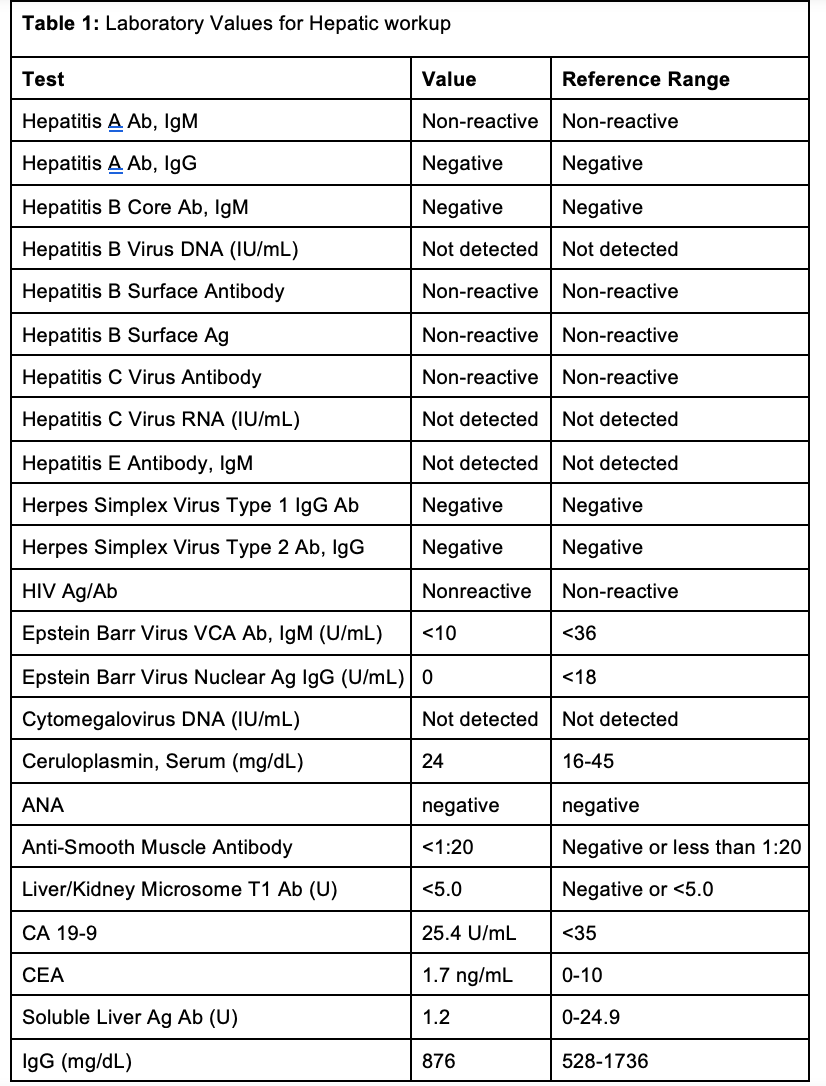
Case Presentation: A 58 year old lady with a history of hypertension and gastroesophageal reflux disease, presented to the emergency department with two weeks of jaundice. Vitals were hemodynamically stable, and she was alert and oriented to person, place, and time. Her physical exam was notable for scleral icterus and jaundice. Laboratory values revealed AST 1527 U/L, ALT 1186 U/L, Alkaline Phosphatase 202, and Total Bilirubin 13.5 mg/dL. Alcohol and acetaminophen levels were undetected. HIV, cytomegalovirus, acute hepatitis serologies, CEA, CA 19-9, Anti-smooth muscle antibody, mitochondrial antibody, immunoglobulin levels, and -1 antitrypsin levels were all unremarkable (Table 1). Medication reconciliation revealed that she has been taking lisinopril 20 mg daily and omeprazole 20 mg twice daily for the past eight months, and subcutaneous semaglutide .25 mg weekly for the past three months. She denied taking any other medications or herbal supplements.While in the hospital, her home medications were held. Abdominal Ultrasound and CT were unremarkable. She underwent a liver biopsy (Figure A) with results showing severe acute hepatitis with significant centrilobular necroinflammation, parenchymal collapse and bridging necrosis, and the absence of plasma cells, mostly compatible with drug induced liver injury (DILI). From thorough medication review it is thought that she had DILI from lisinopril. She was started on prednisone 40 mg daily and instructed to discontinue lisinopril at home. Her liver transaminases and bilirubin decreased sufficiently enough for discharge with outpatient follow up. Outpatient follow up labs showed normalized liver function tests.
Discussion: DILI is a diagnosis of exclusion, and is the most common cause of acute liver failure in the United States (U.S). Here we presented a rare case of lisinopril induced liver injury in a patient with concurrent semaglutide use. There have been few reported cases of lisinopril-induced DILI, with either a hepatocellular or cholestatic pattern. In our patient, the DILI was noted to show a hepatocellular pattern. The classification of the type of DILI can be calculated using the R ratio, which was calculated to be 17.64, indicating a hepatocellular pattern, consistent with pathology. The RUCAM score for our patient was 9, indicating that hepatotoxicity secondary to lisinopril is “highly probable.” Although the direct cause of lisinopril induced DILI remains unclear, there have been some studies that explore possibilities. One potential cause of ACE-inhibitor induced hepatotoxicity could be due the inhibition of bradykinin, which can lead to increased prostaglandin levels, which have been shown to decrease bile flow, explaining possible hepatotoxicity. Although there have not been any reported cases of liver injury due to subcutaneous semaglutide, it is possible that lisinopril and semaglutide may have potentiated each other’s effects. Both drugs inhibit the activation of the nuclear factor (NF)-kB signaling pathway, which could potentially lead to further inflammation and contribute to liver injury. More research needs to be conducted to investigate the interaction between lisinopril and semaglutide.
Conclusions: This case adds to the growing number of reports of lisinopril induced liver injury and suggests a potential drug interaction with semaglutide that may affect liver function. As lisinopril is among the most prescribed drugs in the U.S., it is crucial to raise awareness of this serious, yet rare, side effect.