Background: Factor concentrates such as 4 factor-prothrombin complex concentrate (4F-PCC) are commonly used off-label as reversal agents in factor Xa inhibitor-associated bleeding events. However, there have been limited studies on the efficacy and safety of 4F-PCC use in these settings. We conducted a retrospective observational study of the use of 4F-PCC for major bleeding occurring on apixaban or rivaroxaban and describe dosing, hemostasis, and thrombotic outcomes.
Methods: We identified all 4F-PCC (KCentra) orders administered at a 600-bed academic teaching hospital from March, 2014 to October, 2019 and restricted to patients who received 4F-PCC for apixaban or rivaroxaban reversal. Manual review of medical charts was performed to obtain information on patient and event characteristics, blood product administration, and 30-day post-event outcomes (e.g., hemostasis, thrombosis, and all-cause mortality). We used the International Society of Thrombosis and Hemostasis (ISTH) criteria to define major bleeding events and effective hemostasis.
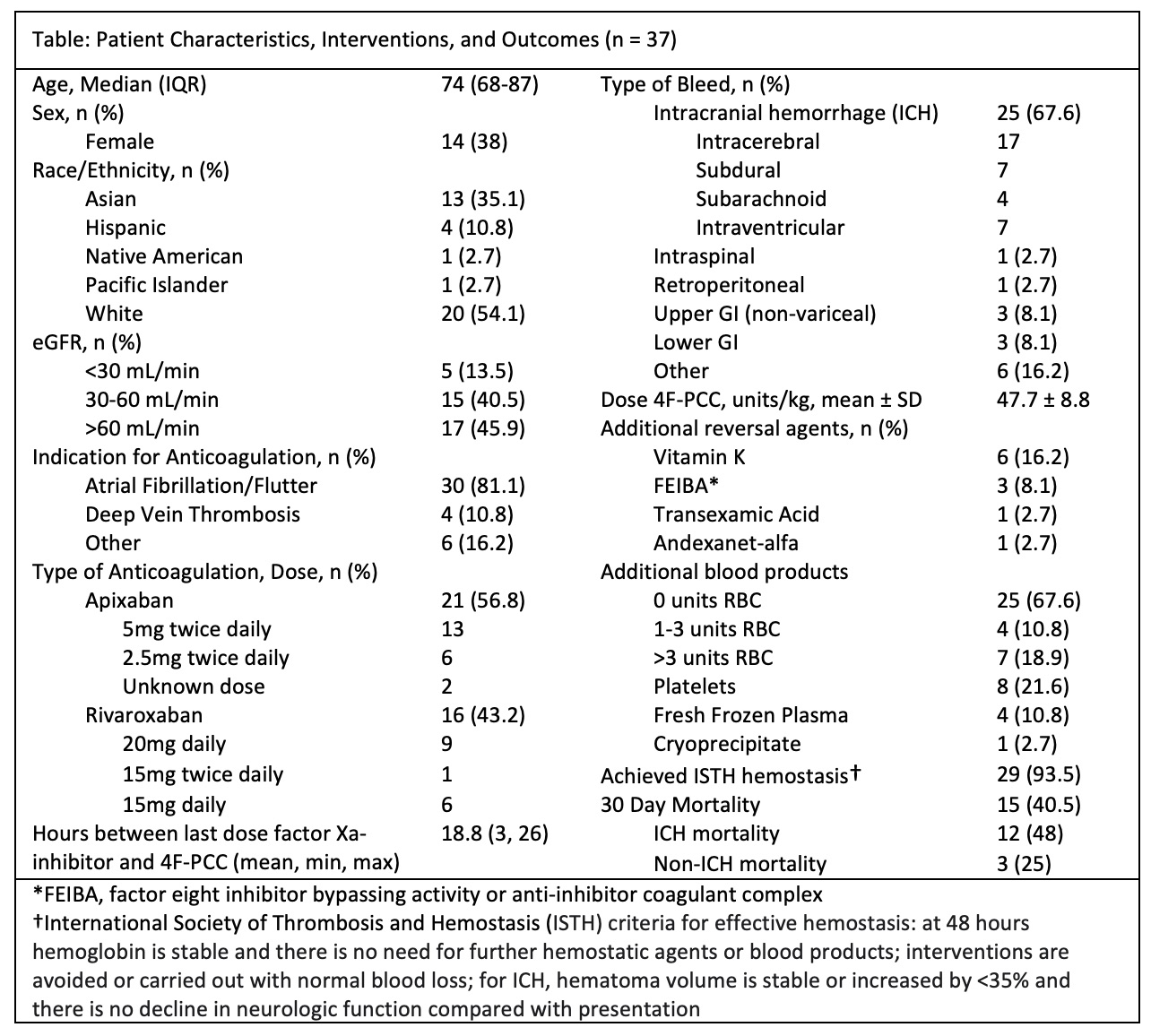
Results: We identified 1796 KCentra orders, with the following reported indications: warfarin bleeding (17%), apixaban/rivaroxaban bleeding (9%), cardiothoracic surgery (48%), and other/unspecified indication (26%). Of the 37 patients given 4F-PCC for factor Xa inhibitor-related bleeding, 35 met ISTH criteria for major bleeding. Intracranial hemorrhage (ICH) was the most common site of bleed, followed by gastrointestinal bleeding. The mean time between the last dose of factor Xa inhibitor and Kcentra administration was 18.8 hours. The average dose of 4F-PCC was 47.7 units per kg patient weight. Hemostasis was achieved in 29 of 37 patients. Overall, 15 (40.5%) patients died within 30 days despite receiving reversal. A greater proportion of patients with ICH died compared with non-ICH bleeds, although the comparison did not reach statistical significance (48% vs 25%, p=.18 by chi-squared test). We identified 1 post-reversal venous thrombotic event (a superficial vein thrombosis) and 1 post-event myocardial infarction within 30 days.
Conclusions: Nine percent of 4F-PCC administered at our hospital was for reversal of factor Xa inhibitors, and primarily for ICH. Nearly half of patients with ICH died despite receiving reversal therapy. Thrombotic complications within 30 days was uncommon. As apixaban and rivaroxaban become increasingly used, it is important to track the management and outcomes of bleeding complications.