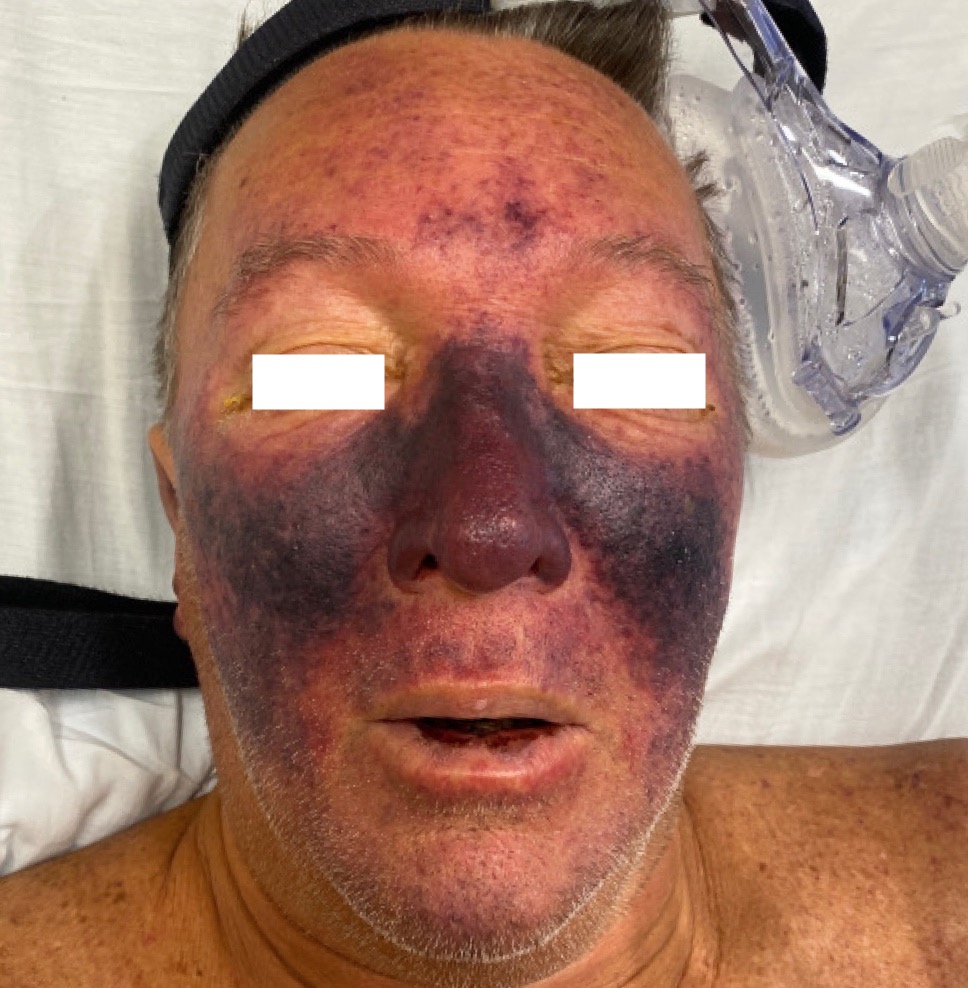
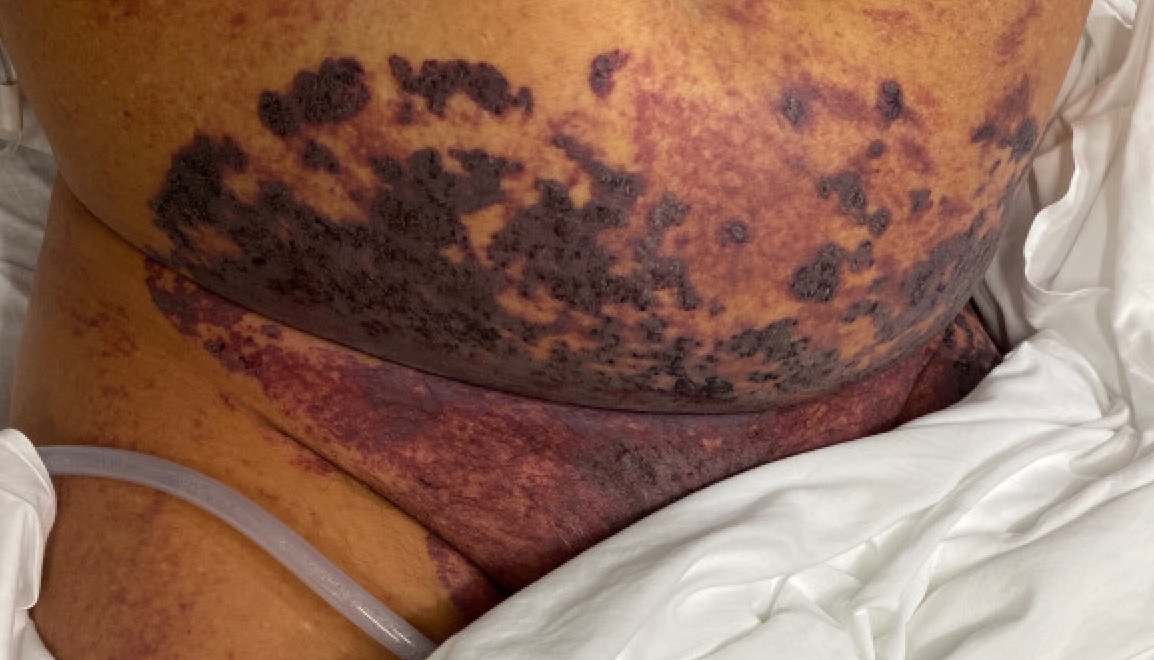
Case Presentation: A 50-year-old male healthcare worker with a past history of splenectomy secondary to EBV complications, mild persistent asthma, and type 2 diabetes mellitus presented to the emergency department with acute hypoxic respiratory failure secondary to COVID-19 pneumonia. He quickly progressed to septic shock with worsening hypotension, tachycardia, lactic acidosis, increasing oxygen demands, and acute renal failure with oliguria. On examination, he had rapidly progressive purple, erythematous, non-blanching, confluent purpura on his face, ears, abdomen, and left leg with cutaneous necrosis which was noted. His labs revealed severe leukocytosis, thrombocytopenia, elevated PT, elevated PTT, elevated D-dimer, and decreased fibrinogen. Peripheral smear showed schistocytes. He was treated with supplemental oxygen (non-invasive ventilation), vasopressors, broad-spectrum antibiotics, fresh frozen plasma, multiple platelet transfusions, and dialytic therapy. Two blood cultures grew viridans group streptococci. He had no history of recent dental surgical procedures, his oral examination was normal, and a transesophageal ECHO was performed that revealed no endocardial vegetations. Our patient surprisingly tested positive for HIV on the last day of his hospitalization and was scheduled for an immediate follow up with an HIV specialist to evaluate his CD4 count, viral load, and antiretroviral therapy options. Ultimately, he was discharged home after a stay of thirteen days.
Discussion: AIPF is a rare complication of sepsis and is commonly associated with bacterial infections, though viral infections have also been reported. The exact pathogenesis of streptococcus AIPF remains unclear but streptococcal pyrogenic exotoxins (speA, speB, and speC) induce cytotoxicity and pyrogenicity, and enhance the lethal effects of endotoxins on the host. This can develop into a cytokine storm and rapidly progress to septic shock, DIC, multi-organ failure, which is associated with high mortality. COVID-19 associated coagulopathy (including DIC and AIPF) is multifactorial – caused as a result of profound inflammatory response from cytokine storm, endothelial damage, and microvascular thrombosis. Another factor increasing our patient’s coagulability was his HIV seropositive status, as chronic immune activation and associated inflammation in HIV increases coagulation risk. Finally, our patient’s asplenic status predisposed him to a higher risk of infections from encapsulated organisms including streptococcus, because of loss of splenic phagocytic function, decreasing serum immunoglobulin levels, suppression of lymphocyte sensitivity, which led to an overwhelming post-splenectomy infection (OPSI).Our patient had a concomitant bacterial (streptococcal spp.) and viral (COVID-19) infection, in the context of his post-splenectomy status and HIV seropositive status – all of which increased the risk of AIFP. Fortunately, our patient recovered with treatment.
Conclusions: Severe COVID-19 infectious are associated with intense inflammation, leading to high rates of thrombotic complications (including AIPF and DIC) which increase morbidity and mortality. Multiple cases of COVID-19 associated AIFP have been reported in the literature, and with the United States reporting more than 14 million cases, hospitalists must carefully consider all the comorbid factors while treating COVID-19 patients as this can guide early interventions and timely treatment.