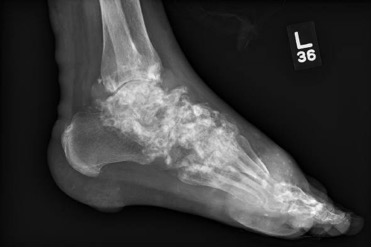
Case Presentation: A 40-year-old man with a history of severe tophaceous gout, not currently on treatment, was directly admitted to the hospital after routine labs showed hypercalcemia (14.1mg/dL) and an elevated creatinine (4.2mg/dL). Vital signs were stable. Exam was significant for large nodules and tophi across most joints, with innumerable subcutaneous nodules embedded in the skin of his arms, legs, flanks and buttocks (Figure 1). The workup of hypercalcemia was systematically approached. First, we determined that the cause was parathyroid-independent, given an appropriately low PTH (11.1 pg/mL). Next, malignancy and granulomatous disease were ruled out with a negative CT scan of the chest, abdomen, and pelvis. A PTH-related protein (PTH-rp), secreted by solid tumor malignancies, and UPEP, SPEP, and light chains, elevated in hematologic malignances, were drawn and all normal. Skin biopsy ruled out cutaneous T-cell lymphoma.Next, TSH and vitamin A level were found to be normal. Finally, a 1,25 dihydroxy-vitamin D was drawn and found to be elevated (85.9 pg/mL), giving us a potential clue. Bilateral hand and foot x-rays showed extensive, calcified tophaceous gout with erosions and bone loss (Figure 2). Skin biopsies showed focal calcifications of amorphous material surrounded by mononuclear histiocytes, giant cells, and fibrosis. After a detailed discussion with consultants, the most likely cause of this patient’s severe hypercalcemia was a chronic granulomatous reaction driven by tophaceous gout.
Discussion: Physiologic calcium homeostasis is largely controlled through the effects of parathyroid hormone (PTH) on the bones, gastrointestinal tract, and kidneys. However, in granulomatous disorders, PTH independent hypercalcemia can occur due to the presence of excess 1α-hydroxylase enzymatic activity in the activated macrophages of granulomas. This allows unregulated hydroxylation of 25-OH-vitamin D, causing elevated 1,25-dihydroxy-vitamin D which functions to increase intestinal absorption and decrease renal excretion of calcium. Hypercalcemia has been documented as a feature of many granulomatous diseases including sarcoidosis, tuberculosis, and Crohn’s disease. While large aggregates of monosodium urate crystals in patients with tophaceous gout can precipitate granuloma formation, there are few documented cases of this process causing hypercalcemia.This patient’s hypercalcemia was a chronic granulomatous reaction driven by their tophaceous gout. In this rare setting, the macrophages surrounding the calcium crystals within their skin triggered a granulomatous reaction. Like sarcoid, these granulomas increase the production of 1-alpha-hydroxylase, which converts 25-OH-vitamin D to 1, 25 vitamin D. This was supported by histiocytes and giant cells seen on skin biopsy. Treatment of the hypercalcemia included intravenous fluid, calcitonin, and prednisone, with the goal of decreasing inflammation surrounding the tophi. Calcium levels and creatinine were restored to normal range and the patient was discharged on allopurinol with a plan of transition to pegloticase, a recombinant uricase, in the outpatient setting.
Conclusions: Though rare, it is important for providers to keep granulomatous reactions as a cause of PTH-independent hypercalcemia and understand it’s unique mechanism. A multi-disciplinary team was instrumental in making the correct diagnosis and is necessary in the workup of future patients.