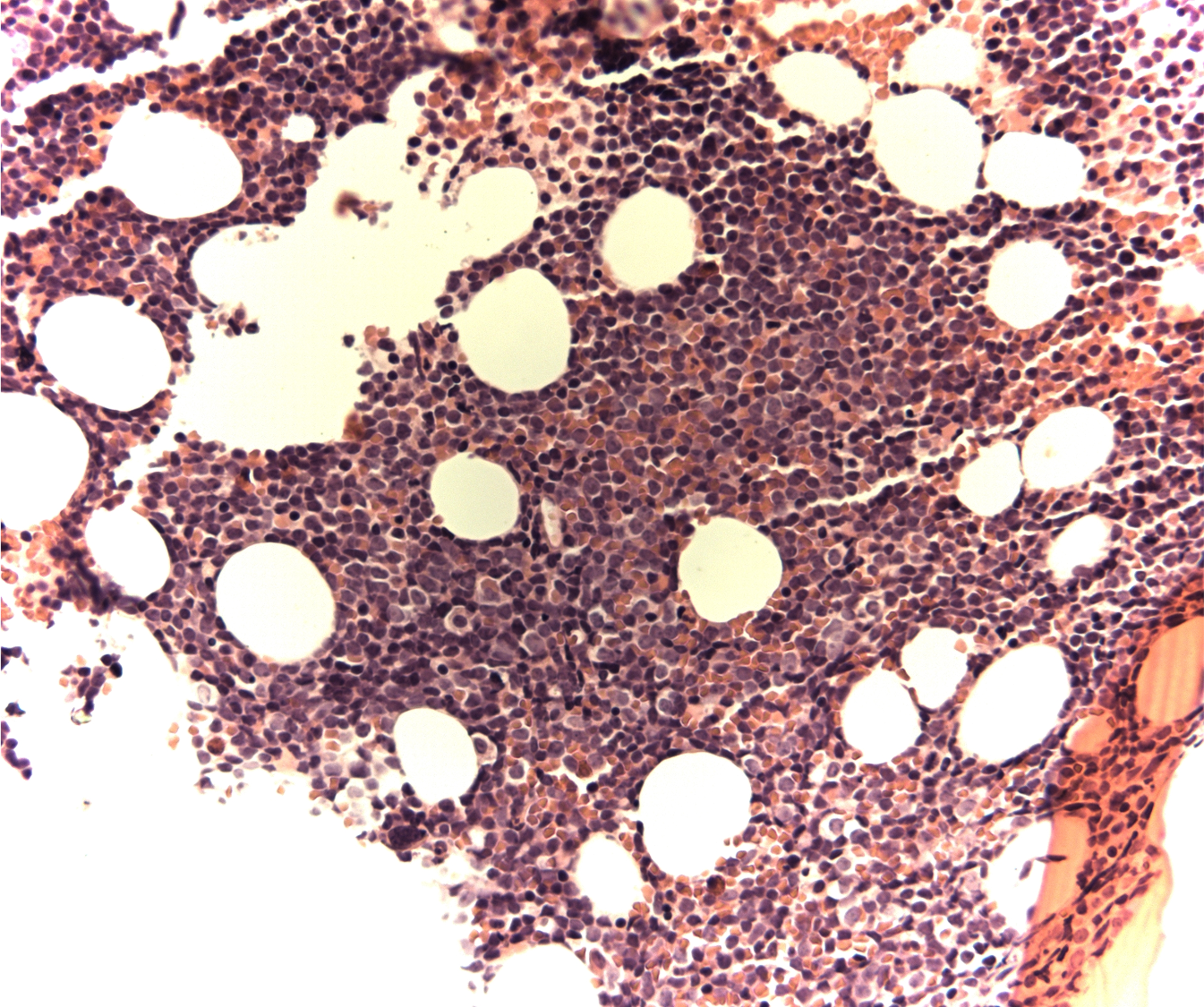
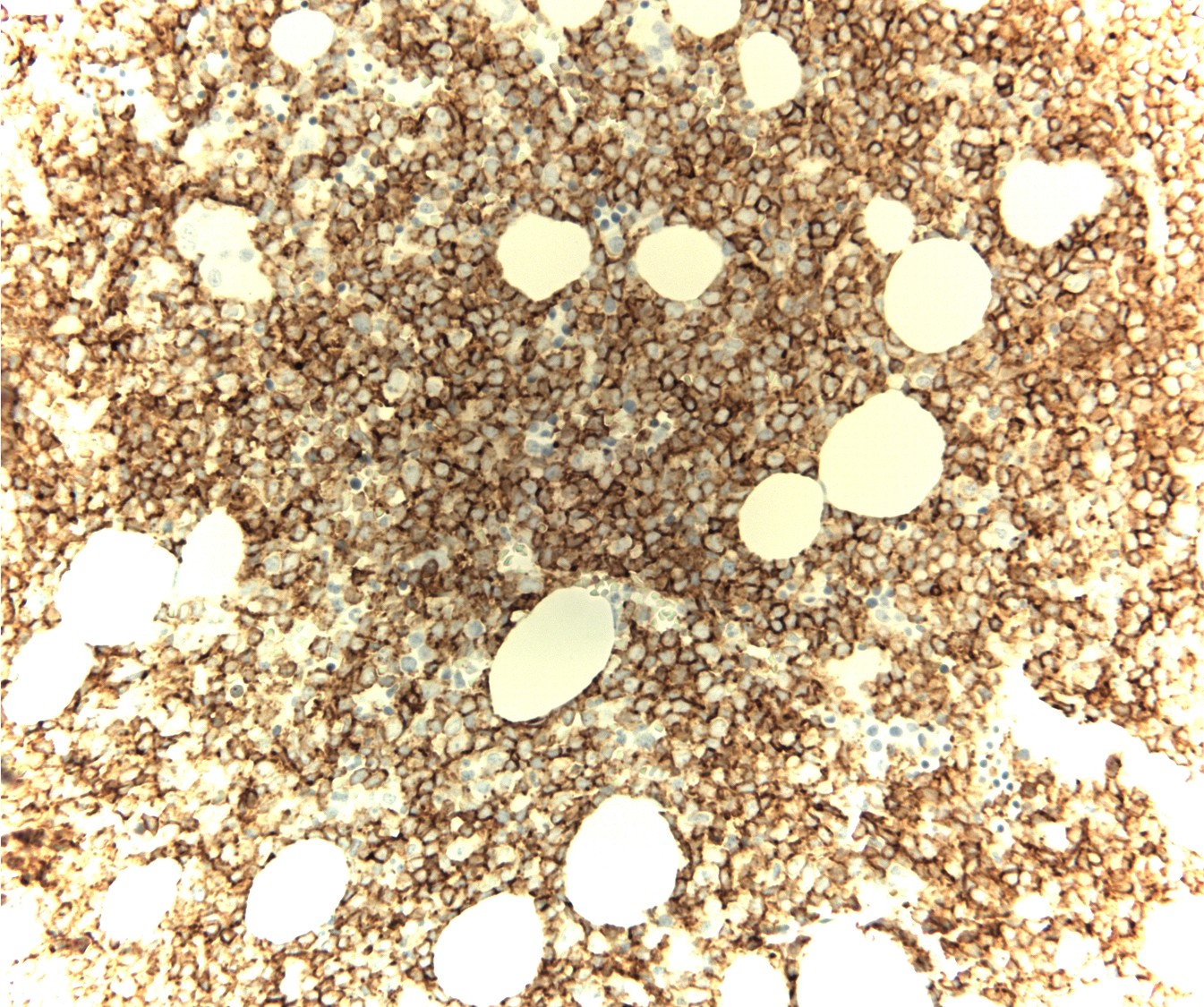
Case Presentation: A 61-year-old man presented to his primary care provider’s office but could not recall reason for his visit. Over the past year, there had been numerous instances of similar forgetfulness, oftentimes upon completing routine activity without additional symptomatology. Medical history was notable for factor V Leiden, hypothyroidism, and transient ischemic attacks. Physical examination was notable for a coarse tremor and subjective sensory loss. No neurological deficits were observed. Laboratory diagnostics showed mild leukocytosis (11.5 k/ul) with borderline basophilia (2.7%). The absolute basophil count was 300. The remainder of the diagnostics were unremarkable except for hyperglycemia (388mg/dL) and a glycosylated hemoglobin of 13.2%. There was no gamma gap. MRI brain and spinal cord as well as CT of chest, abdomen, and pelvis were unremarkable. Lumbar puncture, pursued for further dementia workup, was negative for pleocytosis and malignant cells; there was elevated glucose of 150 mg/dL. An extensive neurological workup was negative for any infectious, paraneoplastic, or metabolic pathology. The patient showed mild improvement with thiamine supplementation, however, his thiamine levels returned normal. In the setting of the noted, albeit slight basophilia, further hematologic workup was pursued during the same admission. Peripheral blood smear showed left shift with bands, metamyelocytes, and myelocytes with immature appearing atypical lymphoid mononuclear cells. Flow cytometry revealed a small population of B-lineage cells suspicious for lymphoblasts with bright uniform CD10 and CD34 expression. Bone marrow biopsy showed a hypercellular marrow with 60% blasts, positive for CD34 and CD10. Chromosome analysis revealed a t(9;22) translocation suggestive of Philadelphia chromosome-positive (Ph+) B-cell acute lymphoblastic leukemia (B-ALL). Subsequently, a malignancy fusion panel demonstrated BCR::ABL1 (e14-e2, e14a2, major, p210), confirming the diagnosis of chronic myelocytic leukemia (CML) in lymphoid blast crisis. The patient was pretreated with steroids and started on dasatinib therapy. He also received intrathecal methotrexate and hydrocortisone for neuroprophylaxis. He had significant improvement thereafter.
Discussion: CML is a malignant neoplasm of hematopoietic cells, typified by a t(9;22) chromosomal translocation, resulting in the formation of the BCR/ABL fusion oncogene. The molecular hallmark of CML is the expression of the p210 oncoprotein due to the Ph translocation. p210 and p190 oncoproteins are the two major isoforms expressed after the Ph translocation. Almost three fourth of the cases of Ph+ B-ALL express the p190 oncoprotein. Our patient, who had phenotypic features of B-ALL, actually had CML in lymphoid blast crisis. In nearly 50% of cases, CML is diagnosed incidentally during routine bloodwork that reveal extremely elevated cell counts. Hyperbasophilia (>1000 basophils per ml) and massive basophilia (>20% basophils) are also common, but neither were present in our patient, thus making it a unique and challenging diagnosis in the setting of neurological symptoms.
Conclusions: CML can present with bizarre symptoms and without leukocytosis. Basophilia may provide a clue. CML in lymphoid blast crisis can be differentiated from Ph+ B-ALL by the expression of p210 oncoprotein.