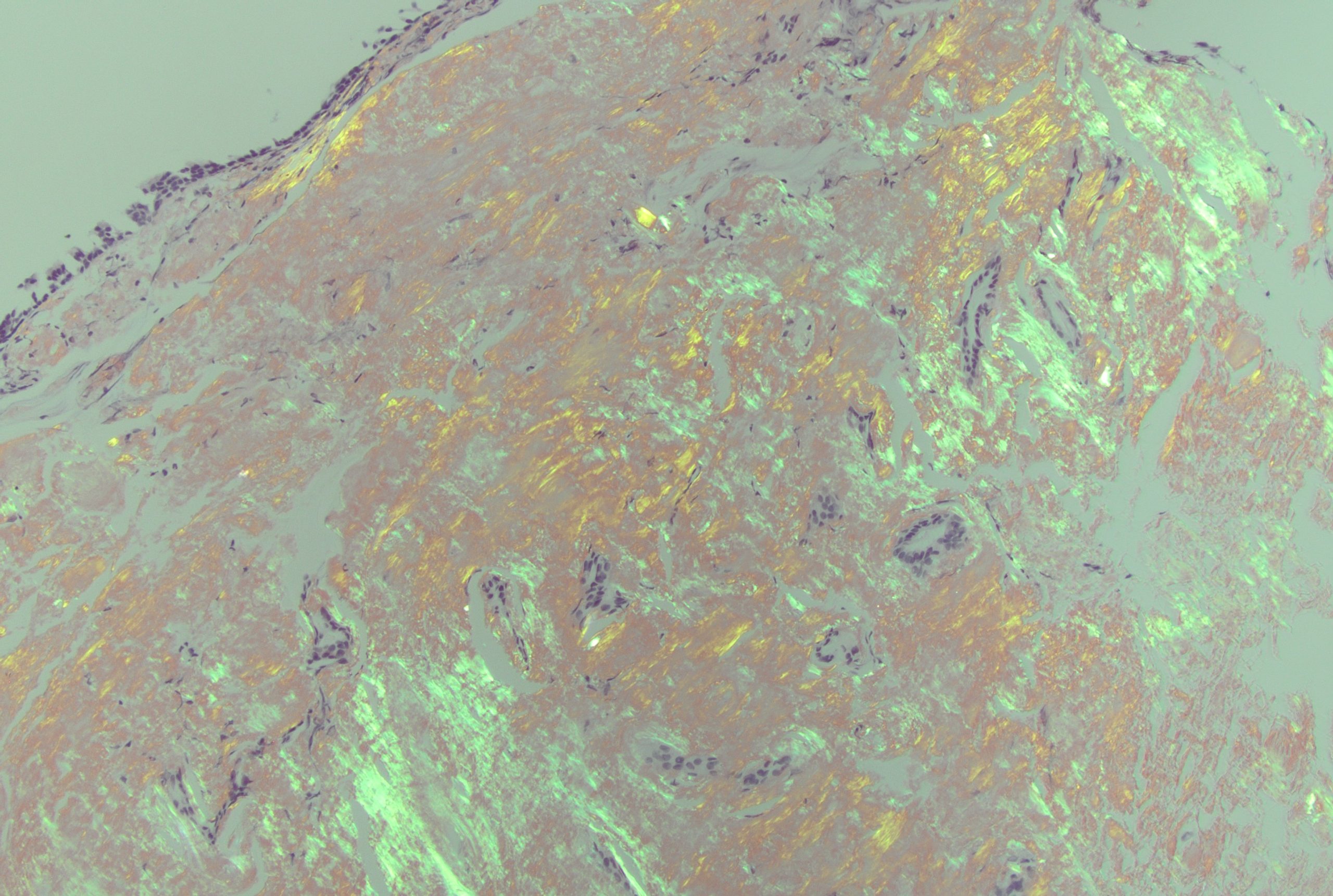
Case Presentation: An 81-year-old male with type 2 diabetes mellitus and emphysema visiting from Thailand presented to the Emergency Department for cough and shortness of breath over one week. Vitals included: 36.3C, 103/87 mmHg, 88 bpm. He was found to be hypoxic with an oxygen saturation of 69% on room air requiring non-invasive ventilation. Computed tomographic (CT) angiography of the chest was negative for acute pulmonary embolus, but notable for large circumferential mass encasing the trachea. Patient underwent bronchoscopy which showed an endobronchial circumferential tumor thickening causing a long stenotic segment at the mid-trachea. This was biopsied in addition to lymph node sampling. Patient underwent three endobronchial debulking and balloon dilation procedures on index admission. Biopsy confirmed diagnosis of AL amyloidosis via histopathology and Congo red staining. Cardiac magnetic resonance imaging (MRI) and CT of the abdomen and pelvis had no evidence of amyloid deposition. He was discharged on room air with outpatient follow-up. However, he presented again with worsening shortness of breath and was re-admitted. Patient was found to have an Aspergillosis tracheitis superinfection, where bronchial washings were positive for Aspergillus antigen as well as by PCR. He remains hospitalized for antifungal treatment and management of his tracheal mass including additional resection and radiation.
Discussion: We present a case of tracheobronchial amyloidosis, a rare variant of AL amyloidosis associated with increased morbidity and mortality due to localized disease burden. Airway involvement in amyloidosis accounts for less than 1% of all benign airway tumors, usually occurs in patients age 40–60 years, with no clear sex predilection.1 Patients present with common respiratory complaints such as shortness of breath, cough, or hoarseness, but can quickly lead to rapid airway compromise as seen in our patient.2 Tracheobronchial amyloidosis may be initially mistaken for other pulmonary pathologies such as asthma, COPD, or pneumonia.3 Our patient initially presented with significant hypoxia and key risk factors such as originating from Southeast Asia and a significant smoking history, as such, a high level of suspicion is required for diagnosis of tracheobronchial amyloidosis. Management of this disease is complex as recurrence is common.4 Due to the friable nature of protein fibril deposition and propensity of amyloid to infiltrate blood vessels, hemorrhage and tissue sloughing during and after debulking is another cause of airway compromise with one case of a patient dying of hemorrhagic shock during an attempted resection.5 Given the local nature of amyloid deposition, debulking procedures and radiation therapy are commonly used, but there is no current definitive management.6 The chance for progression and recurrence is high therefore emphasis should be placed on follow-up for long-term management.7
Conclusions: Risk of airway compromise is extremely high in patients with localized tracheobronchial amyloid disease as it continues to be deposited. Localized amyloidosis warrants accurate diagnosis with computed tomography of the chest, bronchoscopy, and biopsy as well as long-term multidisciplinary care. Additionally, cough and shortness of breath are some of the most common chief complaints of patients presenting to the hospital thus it is important to consider a broad differential diagnosis, especially if patients do not respond to common treatment for pulmonary pathologies.