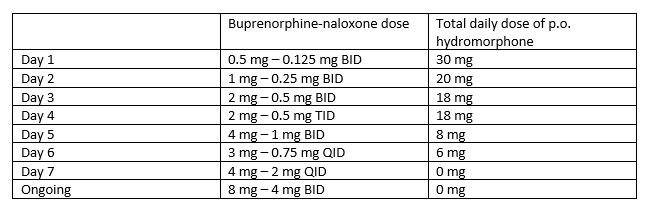
Case Presentation: A 34-year-old male with a history of opioid use disorder (OUD) was admitted after being found down secondary to heroin overdose. He was given 4 mg naloxone in the field with improvement in Glasgow Coma Score from 3 to 12. After naloxone administration, he was tachycardic, tachypneic, hypertensive, and agitated. He reported taking increasing doses of intranasal heroin and hydrocodone/acetaminophen for management of acute right leg pain during the preceding week. Initial labs on admission were consistent with rhabdomyolysis (creatine kinase 52,000 U/L) with concurrent lactic acidosis, acute kidney injury, hyperkalemia, and leukocytosis with neutrophilia. He was treated with aggressive IV fluid. On hospital day two, he developed compartment syndrome with worsening swelling and pain of the right thigh and reduced right foot sensation. He underwent emergent medial and lateral fasciotomies. Postoperatively, he continued to have significant pain requiring high doses of opioid pain medication and was started on hydromorphone patient-controlled analgesia (PCA).During hospitalization, the patient expressed interest in pursuing OUD treatment with buprenorphine and chemical dependency resources. After deescalation to oral hydromorphone, he was initiated on a buprenorphine microdosing protocol to reduce risk for precipitated opioid withdrawal (Table 1). Hydromorphone was tapered and discontinued before discharge. The patient had minimal opioid withdrawal symptoms during induction and was discharged home with arrangements for close chemical dependency follow up.
Discussion: Opioid overdoses make up the highest number of drug overdose deaths in the US and are a significant risk factor for hospital discharge against medical advice. Opioid agonists, including buprenorphine and methadone, are the first-line treatments for OUD. Buprenorphine has a preferred safety profile over methadone given its partial agonist activity at the mu receptor and “ceiling effect” limiting respiratory depression and euphoria. However, given its high affinity for the mu receptor, buprenorphine can precipitate withdrawal when started or dosed concurrently with full opioid agonists. Standard buprenorphine inductions begin with doses of 2-4 mg, require the patient to be in mild to moderate withdrawal, and require abstinence from full agonists for at least 12-24 hours before initiation.In hospitalized patients, the need to utilize full opioid agonists for treatment of severe acute pain can limit buprenorphine induction. Newer low-dose buprenorphine induction regimens, or “microdosing,” have demonstrated reduced risk for opioid withdrawal in patients requiring continued full opioid agonist therapy for pain management. Multiple successful microdosing protocols for treatment of OUD are detailed in the literature, all noting minimal if any opioid withdrawal symptoms in hospitalized patients, as also demonstrated our patient’s case. Microdosing has also shown broad potential for application as a successful bridge to sublingual buprenorphine from heroin, prescription opioids, and methadone. Ultimately, every effort should be made during acute hospitalizations to initiate OUD-directed medications for patients that desire this treatment, and microdosing regimens offer a promising strategy to do so.
Conclusions: Inpatient buprenorphine induction with microdosing is a promising approach to treating opioid use disorder in hospitalized patients concurrently requiring full opioid agonist therapy.