Background: The midline catheter (midline) has emerged as a reliable vascular access device for peripherally compatible infusates. Current guidelines recommend midlines when the intended dwell is ≤14 days since little is known about its safety beyond this period. We sought to describe characteristics and outcomes of midlines that dwell for >14 days.
Methods: Trained abstractors collected information on midline placements in patients admitted to general medical and intensive care units between December 2016 and November 2023 at 67 hospitals participating in the Michigan Hospital Medicine Safety Consortium (HMS). We described patient-, provider- and device-characteristics of longer-dwell midlines (LDM), defined as >14 days dwell, and compared to standard-dwell midlines (SDM), defined as midlines in place for ≤14 days. Patients were followed until device removal, death, or 30-days after device placement, whichever occurred first. Outcomes include major complication (e.g., catheter-related bloodstream infection [CRBSI] or catheter-related venous thromboembolism [CR-VTE]), minor complication (e.g., catheter dislodgement, occlusion, tip migration, infiltration, superficial thrombosis, or exit site problems [including leaking and infection]), and device failure, defined as premature device removal due to any device complication. For comparisons, we used Student’s T-test for continuous variables and Chi-square test for categorical variables. Survival models were run while adjusting for prespecified covariates.
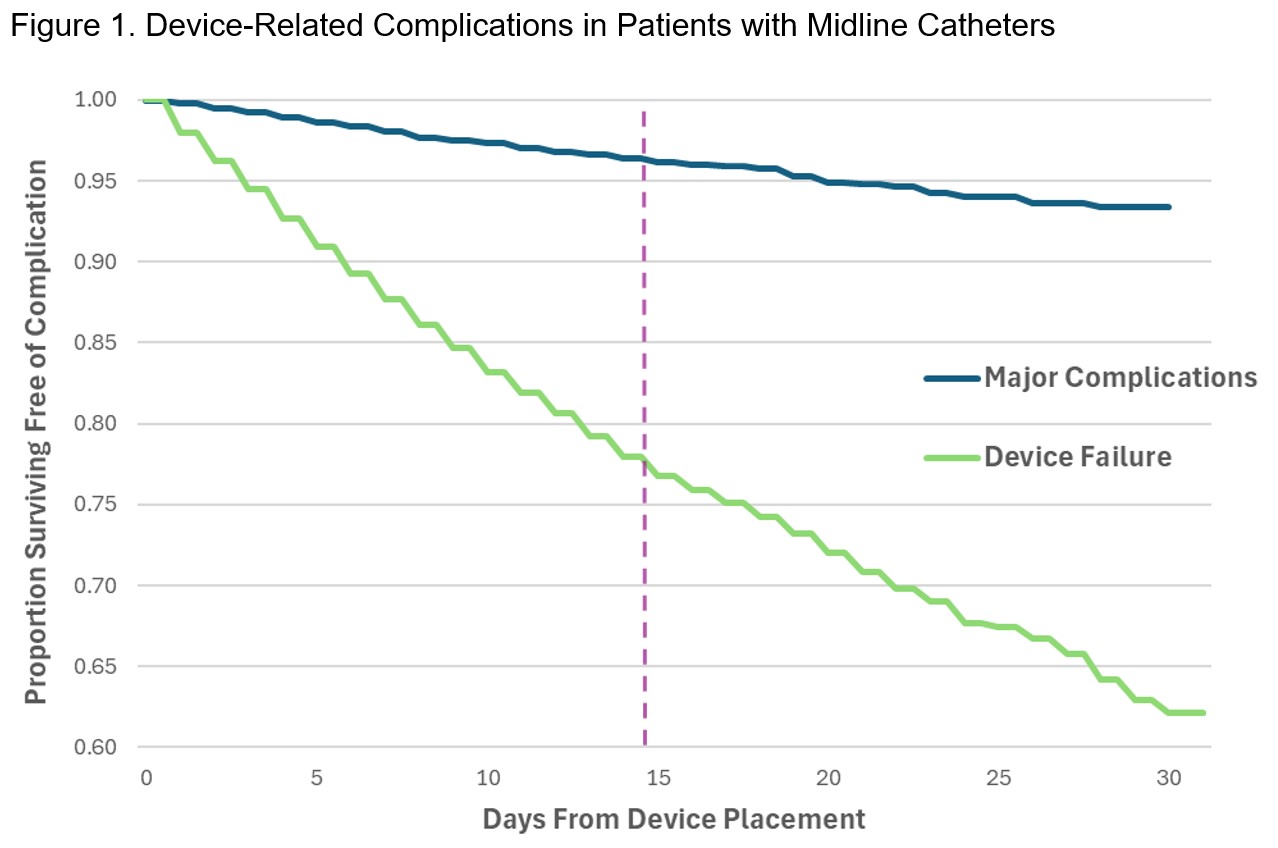
Results: Of 27,300 midlines analyzed, 3,183 (11.7%) dwelled for >14 days and 24,117 (88.3%) for ≤14 days. Median dwell was 20 days (Interquartile range [IQR], 17-25 days) for LDM and 5 days (IQR, 3-8 days) for SDM. Most common indications for LDM were antibiotics (56.9%) and difficult access (36.2%). Major complications occurred in 2.7% of patients with LDM, and in 1.4% of those with SDM, p<.001. CRBSI was observed in 0.6% of LDM and 0.2% of SDM, p<.001; while CR-VTE was seen in 2.1% of patients with LDM and 1.2% of those with SDM, p<.001. However, LDM had lower rates of minor complications (11.6% vs. 13.1%, p=.02) and device failure (9.4% vs 12.5%, p<.001). Adjusted survival plots showed the probability of surviving free of major complications was 97% at 14 days and 93% at 30 days, while probability of freedom from device failure was 78% at 14 days and 62% at 30 days.
Conclusions: Midlines dwelling longer than 14 days are common and majority are placed for antibiotic infusion. Longer dwell midlines appear to have higher rates of major device complications, but lower rates of minor complications and device failure compared to standard dwell midlines. Future studies should account for the potential effect of specific infusates on these outcomes.