Background: Proton Pump Inhibitors are widely misused in both the outpatient and inpatient setting. Often, they are initiated and continued for reasons that do not follow best practice guidelines.
Purpose: A Proton Pump Inhibitor Stewardship Committee was created to address this problem as part of a Quality Improvement project. The goal was to determine the incidence of inappropriate stress ulcer prophylaxis, identify opportunities for interventions, and assess the impact of the interventions.
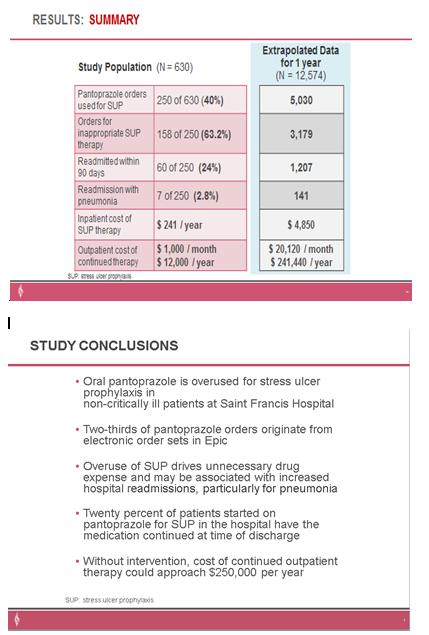
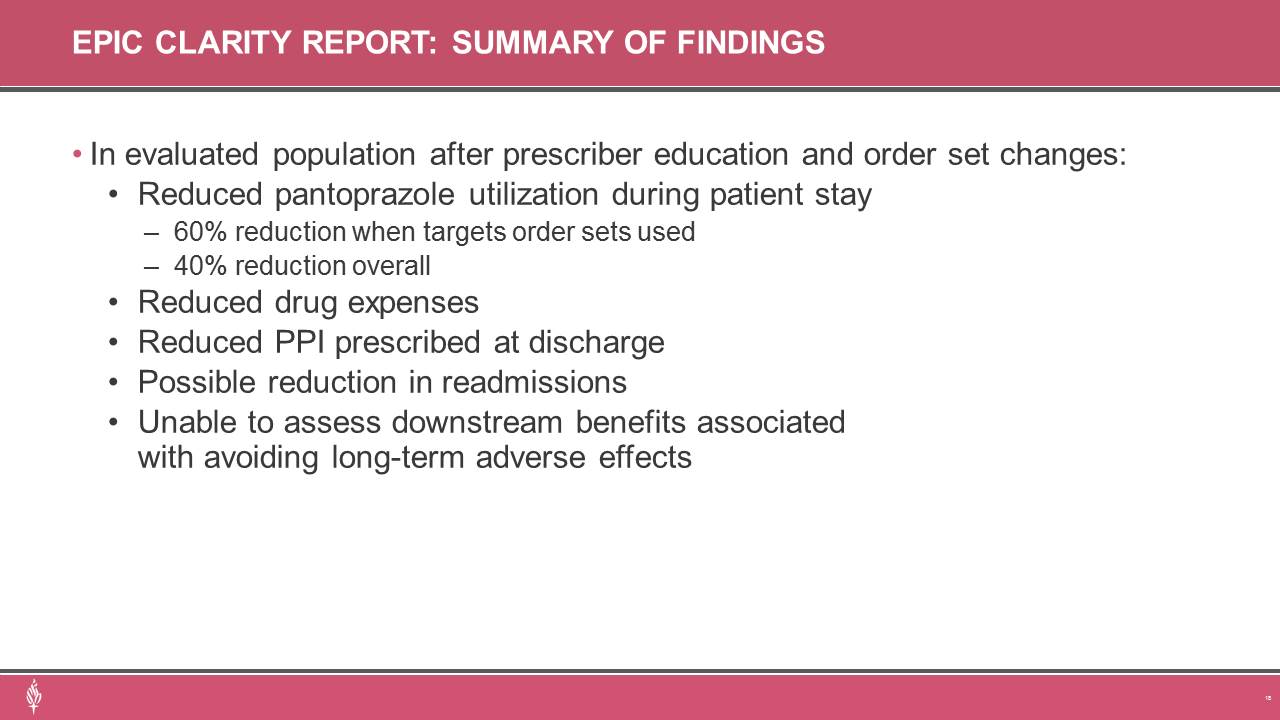
Description: This two-phase, before-and-after, quality project was conducted at the flagship facility of a multi-hospital health system. Phase one was a randomized, retrospective chart review of hospitalized adult patients admitted between February 2020 and January 2021. Patients were eligible for inclusion if they were administered routine doses of oral Pantoprazole for stress ulcer prophylaxis while admitted to a non-intensive care unit of the hospital. Excluded patients were those who had proton-pump inhibitors prescribed prior to hospital admission or ordered by a gastroenterologist, ICU admission, active GI bleeding, a documented indication for acid suppression treatment or high-risk factor for developing stress ulcers. A follow-up evaluation was conducted to assess the efficacy of stewardship interventions, including provider education and order set revisions. Phase two was a retrospective analysis of electronic medical record data for all patients that received Pantoprazole. Data was extracted using EMR generated reports designed to best match phase one eligibility criteria to compare phase one data to Pantoprazole utilization in patients admitted between February and July 2022. The primary outcome was the incidence of inappropriate stress ulcer prophylaxis (SUP). Secondary outcomes included use of electronic order sets, continuation of therapy at discharge, cost of inappropriate therapy, and hospital readmissions. SUP was deemed appropriate for patients with coagulopathy, chronic liver disease, history of GI ulcers/bleed, guaiac positive stools, high-dose daily corticosteroids, major surgery, anticoagulation treatment, or antiplatelet therapy.Results:In phase one, 630 patients were evaluated, with 380 excluded, to achieve the targeted 250 included patients. SUP was appropriate in 36.8% of patients. Two-thirds of orders originated from an electronic order set, and 20% of patients had therapy continued when discharged from hospital stay. Ninety-day readmission rate was 24% (60 patients). An annualized inpatient drug cost of $4,850 was contributed to inappropriate SUP. Extrapolated cost of outpatient drug therapy for health system patients that continued inappropriate therapy for 12 months was estimated at $241,000.Phase two data revealed an overall 40% reduction in patient admissions with Pantoprazole administration (reduced from 25% in phase one to 15%). A 63% reduction was observed in admissions where targeted electronic order sets were utilized. Decreased utilization also translated to a reduction in inappropriate therapy being continued upon hospital discharge and reduction in estimated outpatient drug costs of $30,000 per year. A downward trend in 30-day hospital readmission rate was noted (13.4% to 13%).
Conclusions: The conclusion was Proton Pump Inhibitors were misused for GI prophylaxis. In response a PPI stewardship committee was formed and implemented education and order set revisions. This resulted in decreasing the misuse of Proton Pump Inhibitors for GI prophylaxis..