Case Presentation: This is a 46-year-old man with poorly controlled diabetes mellitus, hypertension, and hyperlipidemia who presented with acute worsening abdominal pain for two days, along with nausea, vomiting and anorexia. The patient had polyuria and polydipsia but denied dysuria, diarrhea, fevers or chills. He had no personal or family history of pancreatitis, gallstones, or abdominal surgeries. He also denied alcohol misuse and smoking. Home medications included, metformin and semaglutide. His endocrinologist added empagliflozin 6 days prior to symptoms onset. No other home medication known to cause pancreatitis was identified. On presentation, he was tachycardic and tachypneic but normotensive. His abdomen was soft, non-distended, and minimally tender in the epigastric region. Pertinent laboratory data included venous blood gas pH 6.86, bicarbonate <5, serum anion gap >38, serum glucose 662, urine ketones 2+, serum calcium 9.8, lactic acid 9.7, lipase 381, and serum triglycerides 196. Also, WBC 36.7 with 8% bands, and blood and urine cultures were negative. Abdominal ultrasound had no evidence of common biliary duct dilatation, cholelithiasis, or acute cholecystitis. He was diagnosed with diabetic ketoacidosis due to acute pancreatitis, and admitted to the ICU. Metformin and empaglifozin were held on admission. He improved with medical management without further complications and was discharged home on day 3 of admission.
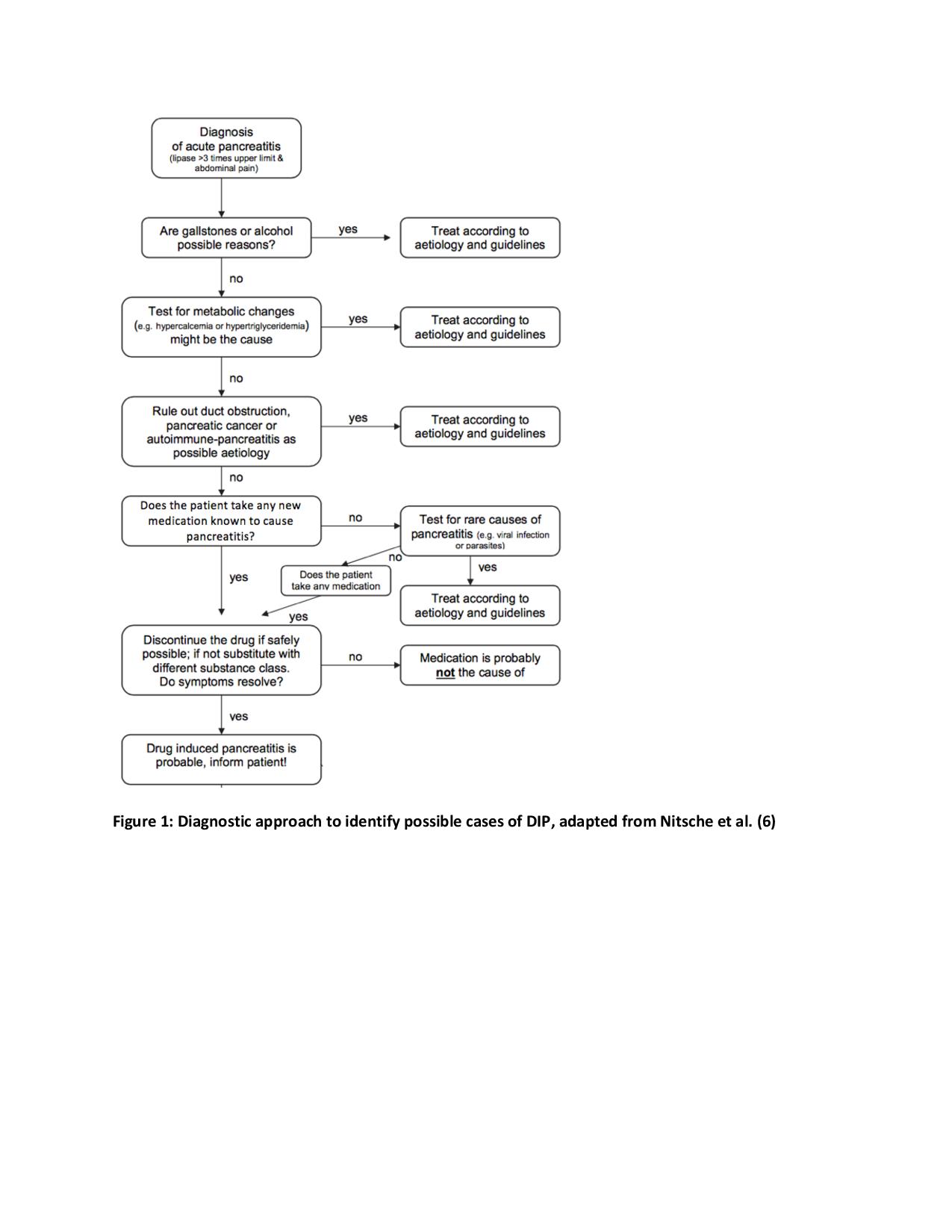
Discussion: Empagliflozin is a new class of sodium glucose co-transporter-2 (SGLT2) inhibitor. Reports to the Food and Drug Administration suggest that SGLT2 inhibitors may be associated with acute pancreatitis, however, clinical trials have been too small to appropriately assess this potential adverse event. Four case reports identifying drug induced pancreatitis (DIP) secondary to canagliflozin, and only two cases secondary to empagliflozin have been published. This case report likely represents a case of empagliflozin-induced pancreatitis. The presence of abdominal pain with an elevated lipase three times the upper normal limit, supports the diagnosis of acute pancreatitis. The diagnosis of DIP is mostly a diagnosis of exclusion in the setting of convincing drug exposure. In our case, gallstone pancreatitis was ruled out given the absence of gallstones or biliary dilatation on abdominal ultrasound; alcoholic pancreatitis was unlikely given a non-existent history of alcohol use; serum triglycerides were close to normal limits, making hypertriglyceridemia-induced pancreatitis unlikely. The timing between starting empagliflozin and symptom onset suggests empagliflozin-induced pancreatitis. Although the patient was also taking metformin, a known cause of pancreatitis, this was a chronic medication, and no history of similar complaints was noted.
Conclusions: This case report most likely represents a rare case of Empagliflozin-induced pancreatitis. Although DIP is a rare adverse reaction to SGLT2 inhibitors, it is prudent that clinicians be aware and watchful for this severe adverse effect as the use of SGLT2 inhibitors increase.