Case Presentation:
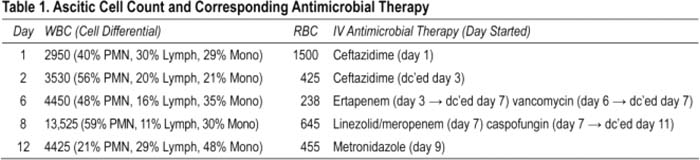
A 34‐year‐old man with end‐stage renal disease on hemodialysis and cirrhosis presented with 2 months of progressive abdominal distension and diffuse pain. He denied fever and had an otherwise negative review of symptoms. He was found to have new‐onset ascites with a senjm‐ascites albumin gradient (SAAG) of 0.7 g/dL and presumed spontaneous bacterial peritonitis (SBP) by cell count and started on ceftazidime. Because of rapidly reaccumulating ascites and persistent abdominal pain, he underwent repeat paracentesis on days 2, 6, and 8 (Table 1). Surprisingly these revealed worsening leukocytosis despite him remaining afebrile, hemodynamically stable, without peritoneal signs, and with negative ascitic and blood cultures. He had no risk factors for tuberculosis, malignancy, or an autoimmune disorder and was PPD‐ and HIV‐negative. All other ascitic studies were unremarkable, including fungal and AFB cultures, adenosine deaminase, amylase, triglycerides, tumor markers, and cytology. Serial imaging revealed stable peritoneal enhancement consistent with peritonitis but no acute intra‐abdominal process or evidence of secondary peritonitis. On day 9. he developed profuse watery diarrhea. Clostridium difficile toxin and somatic antigen assays returned positive. He was treated with metronidazole, and his abdominal pain rapidly resolved within several days. Paracentesis on day 12 finally documented a decreasing ascitic leukocytosis, and his ascites also ultimately resolved; other antimicrobials were discontinued. Based on clinical features, he was diagnosed with C. difficile—associated culture‐negative neulrocytic ascites and discharged home to complete a 2‐week course of metronidazole.
Discussion:
Hospitalists may be faced with cullure‐negalive neulrocytic ascites, a potential diagnostic dilemma in hospitalized medical patients. It is a variant of SBP and characterized by an ascitic neutrophil count >250/mm3, negative ascitic culture, and absence of both an intra‐abdominal infection and antimicrobial therapy in the preceding 30 days. When combined with a low SAAG, the differential diagnosis includes chronic granulomatous infections, malignancy, and pancreatic, biliary, or nephrogenic ascites. C. difficile has also been reported as a distinct etiology, particularly in cirrhotics and the immunocompromised. A proposed pathophysiologic mechanism is toxin‐mediated vascular permeability facilitating bacterial translocation. As C. difficile becomes more prevalent, hospitalisls need to be aware of neutrocytic ascites as a potential complication.
Conclusions:
Clostridium difficile can cause culture‐negative neutrocytic ascites in hospitalized medical patients and should be considered when standard infectious evaluations are unrevealing. particularly when the ascitic leukocytosis rapidly rises.
Author Disclosure:
A. Lai, none.