Background: Failure to follow-up results of laboratory tests pending at discharge (TPADs) can lead to patient harm. Numerous interventions have been proposed to improve follow-up. The Laboratory Medicine Best Practices (LMBP™) workgroup, sponsored by the Centers for Disease Control, commissioned a systematic review to address the impact of various interventions on TPAD documentation, communication, and adverse event rates, including readmission and emergency department (ED) visits. The goal of this systematic review was to identify and appraise interventions that address TPADs and, if possible, recommend best practices to reduce patient harm.
Methods: All studies published in English from 2005 to present were eligible for inclusion. Studies of patients discharged from an inpatient facility or ED were identified via searches of PubMed, Embase, Cochrane, Web of Science, and CINAHL using a list of search terms and medical subject headings (MeSH), as applicable. A junior abstractor performed the primary abstraction, which was reviewed by a senior researcher. The LMBP™ A-6 methodology was used to assess study characteristics, practice description, outcome measures, and results. Strength of evidence ratings were assigned using a cumulative scoring system based on the number of studies addressing each question, effect sizes, and study quality.
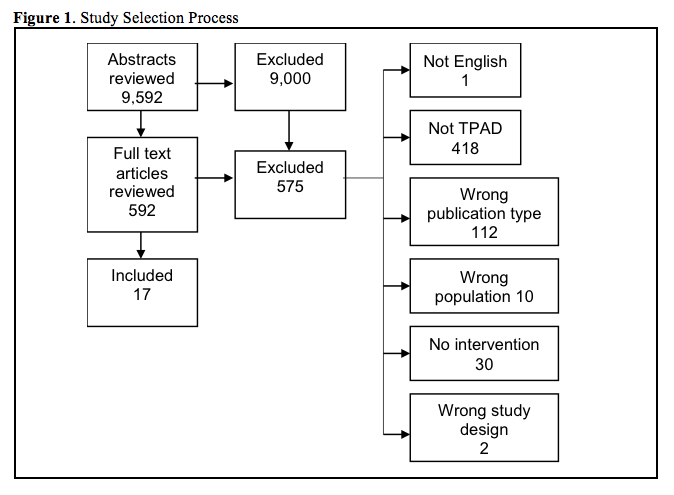
Results: In total, 9,592 references and 592 full text references were reviewed. Seventeen references met inclusion criteria. Three studies addressed the effect of automated notification on the proportion of patients without timely TPAD follow-up. There is insufficient evidence that these interventions reduce the proportion of patients without timely follow-up. Ten studies addressed the effect of automated notification systems or provider education on TPAD documentation and communication with outpatient providers. There is moderate evidence that automated interventions can improve discharge documentation and suggestive evidence that automated alerts may improve physician awareness of TPAD results. Four studies addressed the effect of automated notification or case management interventions on adverse event rates. There is insufficient evidence that these interventions reduce adverse events.
Conclusions: This systematic review finds moderate evidence supporting use of automated tools to identify TPADs for inclusion in discharge summaries to promote documentation and communication with ambulatory providers. There is suggestive evidence that automated alerts to a designated responsible physician can improve awareness of TPAD results. While additional evidence emerges, hospitals and patients may benefit from leveraging technology to automate alerts for TPAD results, which may mitigate harm related to inadequate follow-up.