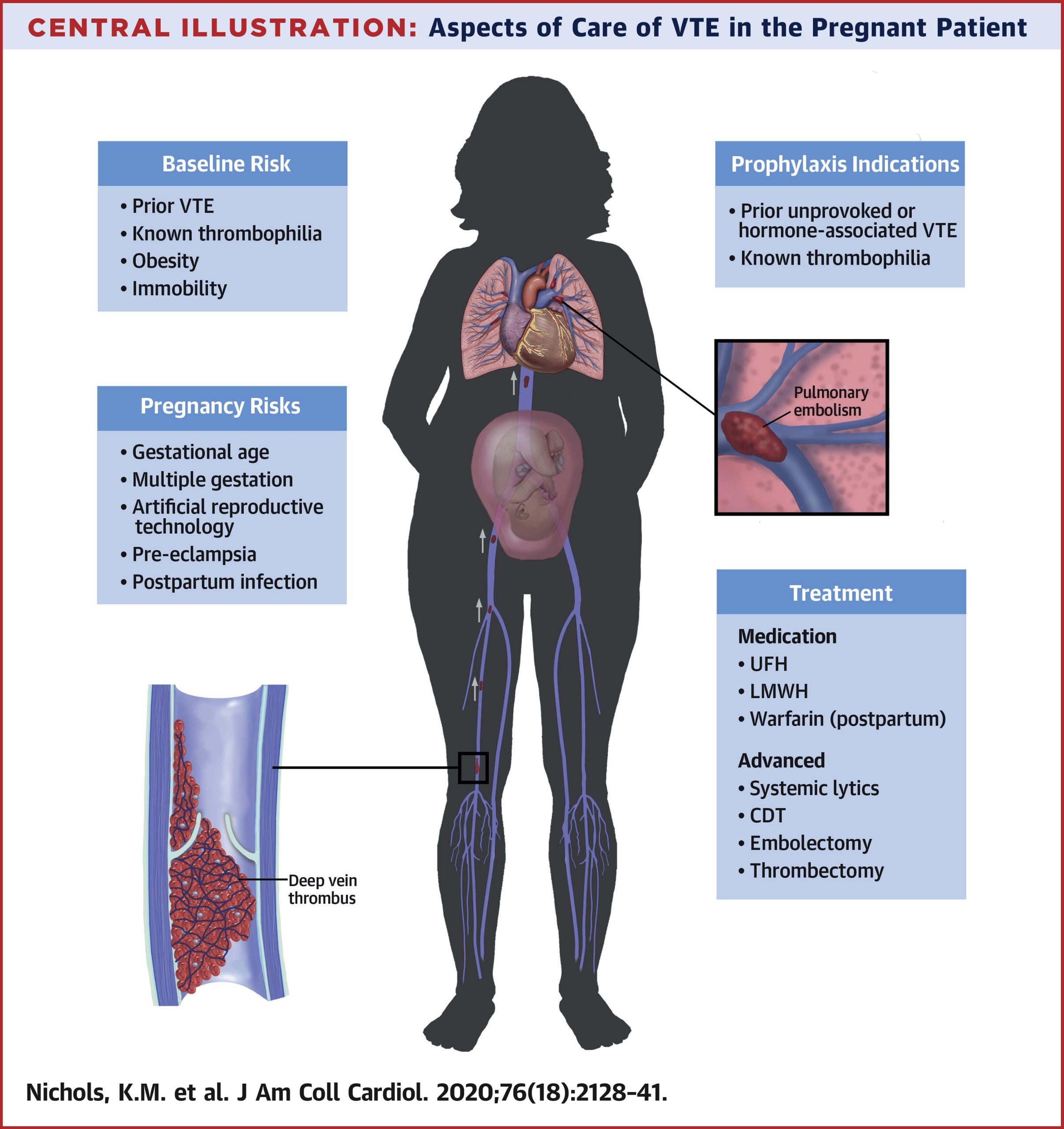
Case Presentation: A 20-year-old female, nonsmoker, G1P0 at 25 weeks, presented to the ED with left leg swelling. Ultrasound confirmed a left femoral DVT. CBC and CMP were unremarkable. She started enoxaparin 80mg twice daily and followed with OB/GYN. Her hypercoagulable workup showed a prolonged aPTT and DRVVT, which corrected with mixing, indicating absence of lupus anticoagulant. Anti-cardiolipin IgG and beta-2-glycoprotein IgG antibodies were elevated. Given her DVT and the presence of these autoantibodies, she has features of probable antiphospholipid antibody syndrome (APS). After 8 weeks, she presented to the ED with right leg discomfort. She was adherent to her enoxaparin injections. Ultrasound revealed right femoral DVT; V/Q scan was negative for pulmonary embolism. CBC and CMP were normal. Maternal-fetal medicine and hematology-oncology services were consulted. Anti-factor Xa levels were obtained to assess her therapeutic index on the current enoxaparin regimen; the results were subtherapeutic. Her dose was increased to 100mg twice daily with anti-factor Xa levels reaching therapeutic levels thereafter. At 39 weeks, she had an uneventful delivery of a healthy male, with plans to reassess autoantibody levels at follow-up.
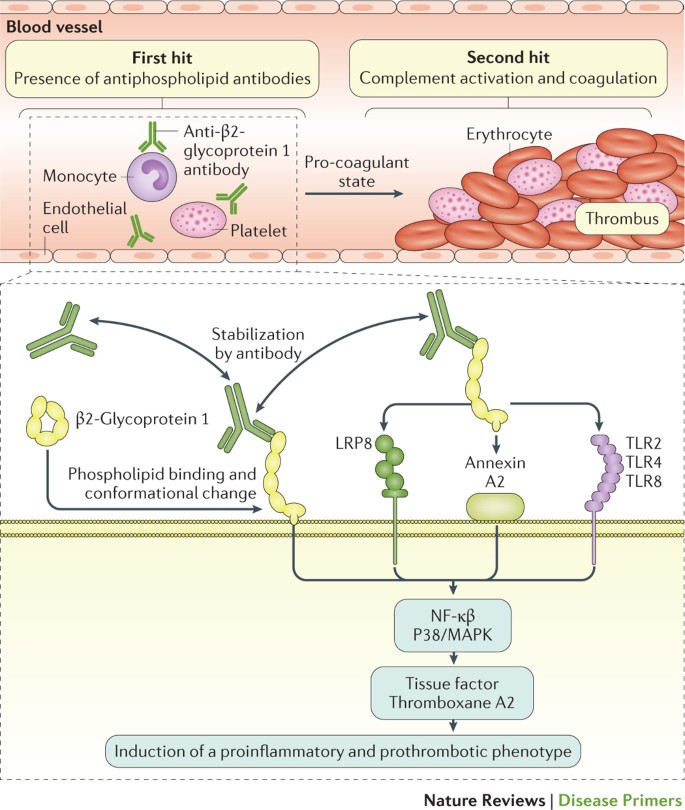
Discussion: APS is an autoimmune condition characterized by antiphospholipid antibodies (aPLs) combined with venous or arterial thrombosis. The most significant aPLs contributing to the hypercoagulable state are anti-cardiolipin, anti-beta-2 glycoprotein-I, and lupus anticoagulant. aPLs interfere with natural anticoagulation by inhibiting protein C and antithrombin, activating the complement cascade, and preventing fibrinolysis. In obstetric APS, trophoblasts express beta-2 glycoprotein-I, which triggers an IL-8, MCP-1, GRO-α, and IL-1β-mediated inflammatory response that induces complement on trophoblasts to cause direct fetal tissue damage. This is a primary target for therapy with unfractionated or low molecular weight heparin, which has been hypothesized to attenuate inflammation-mediated complement activators. Anticoagulants without anti-complement properties—e.g., fondaparinux—are ineffective therapies in APS. PT and aPTT can be artificially prolonged and unreliable. Anti-factor Xa activity more accurately reflects therapeutic heparin effect for monitoring.
Conclusions: Our case highlights the complexity of diagnosis and management of APS. A high-risk APS profile is defined by the presence of lupus anticoagulant and/or the presence of both anti-cardiolipin and anti-beta-2 glycoprotein-I. These autoantibodies can be transient and should be reassessed. With the high risk of pregnancy loss, awareness of treatment and monitoring strategies is imperative, and an interdisciplinary approach may be useful. In this case of probable thrombotic APS, treatment for obstetric APS was pursued due to the morbidity associated with circulating aPLs. Monitoring treatment response with anti-factor Xa levels is unconventional compared to non-pregnant patients .