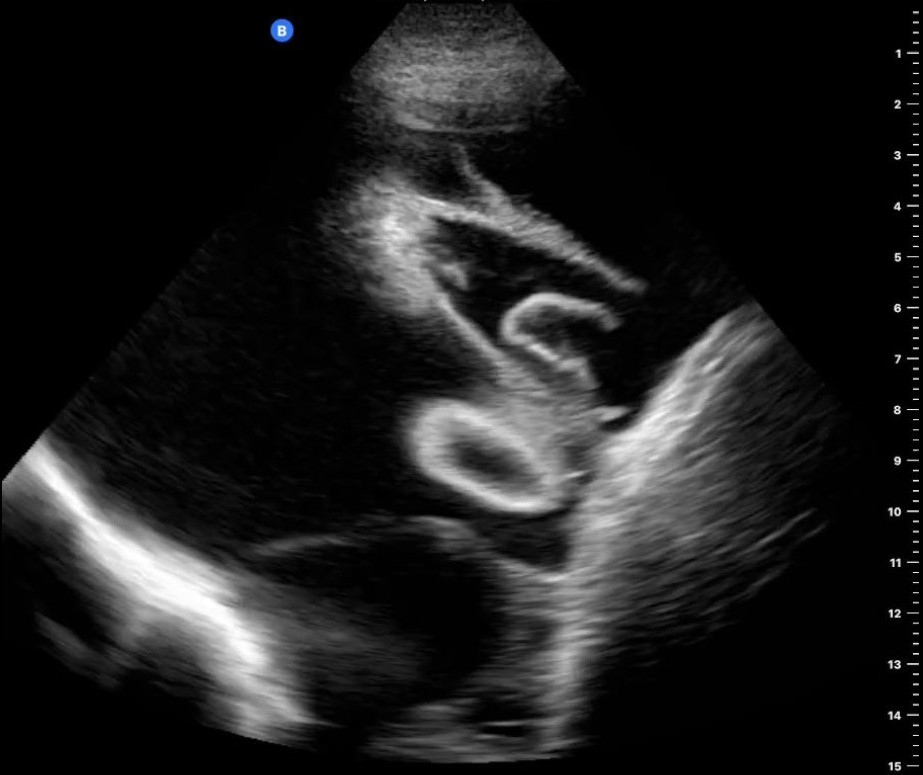
Case Presentation: A 43-year-old male with glucose-6-phosphate dehydrogenase deficiency and human immunodeficiency virus (HIV) infection presented to the emergency department with subacute onset cough and shortness of breath. Medical history was significant for development of HIV after failed post-exposure prophylaxis with brief achievement of undetectable viral load on antiretroviral therapy (ART) followed by inability to continue treatment and loss to follow-up due to financial barriers. On arrival he was hemodynamically stable but tachypneic with SpO2 92% on ambient air. His workup was significant for positive HIV antibody, CD4 count 39 with 3% concordance, and positive Epstein Barr Virus (EBV) PCR. CT revealed patchy nodular and consolidative opacities throughout both lungs with large bilateral pleural effusions. Ultrasound redemonstrated bilateral pleural effusions that appeared complex with septations (figure 1). A diagnostic and therapeutic thoracentesis was performed, which showed yellow cloudy pleural fluid with 1,928 nucleated cells (81% “malignant lymphoma cells”), pH 7.56, LDH 1,600, protein 5.3, and glucose 58. Bronchoscopy demonstrated numerous red lesions consistent with Kaposi sarcoma (KS) (punch biopsy of shin lesion confirmed KS), and BAL studies were positive for pneumocystis pneumonia.Pleural fluid cytology revealed numerous large and atypical pleomorphic lymphocytes positive for CD30, human herpesvirus 8 (HHV8), and EBV, establishing a diagnosis of primary effusion lymphoma (PEL). Subsequent PET scan revealed avid disease in lymph nodes in neck, chest, abdomen, and pelvis. The patient was initiated on chemotherapy (dose adjusted-EPOCH) with a plan to start ART after 2 weeks of treatment.
Discussion: PEL is a rare disease accounting for just 1-4% of HIV-related lymphomas (1-3). While most often diagnosed in patients with HIV, it has also been described in patients following solid organ transplant and chronic hepatitis C (4-8). The pathogenesis is related to HHV8 infection, so patients may present with other sequelae of HHV8 such as KS as was seen in this patient (9,10-12). EBV is also often implicated, though its role in the development of PEL is less defined (13,14). PEL may develop on a variety of serosal surfaces but is most often seen on the pleura, pericardium, and peritoneum, so patients most often present with signs and symptoms of fluid buildup in these spaces (1,9,15,16). Examination of fluid often reveals an abundance of malignant cells and an exudative and sometimes bloody effusion (17). Ultimately, diagnosis of PEL is confirmed by the presence of HHV8 in the nuclei of malignant cells. The overall prognosis of PEL is poor, with patients living an average of 2-3 months without treatment (18,19). Treatment consists of a combination of ART, chemotherapy, radiation, and antiviral therapy, often in a clinical trial setting due to its rarity. Even with treatment, the prognosis is poor with an average survival of 6 months (9,10,16,18,19).
Conclusions: PEL is a rare subtype of HIV-related lymphomas associated with HHV8 infection, as demonstrated in this case. Unfortunately, there is little data to guide therapy, so most clinicians are informed by case reports and expert opinion. Even with therapy, prognosis is still very poor. This demonstrates a need for awareness and prompt enrollment of patients in clinical trials to learn more about this rare disease and to help define future treatment protocols.