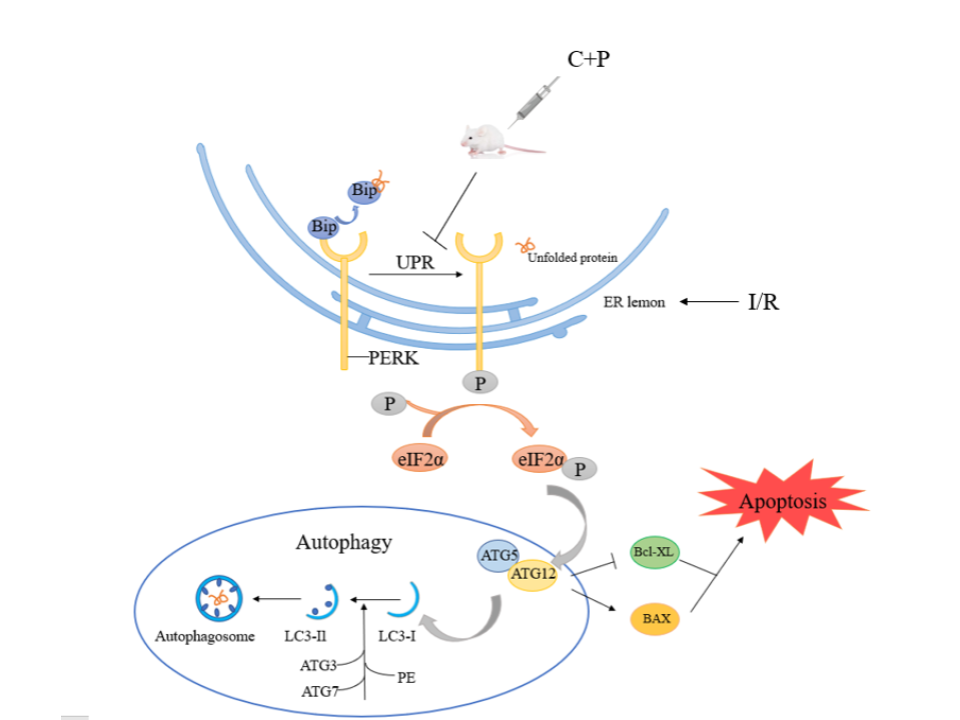
Background: Stroke is the leading cause of mortality worldwide. However, available treatment options are limited due to the narrow treatment time window and reperfusion injury after revascularization. New treatment modalities to overcome these challenges are in need. Chlorpromazine and promethazine (C+P) have been utilized for cerebral edema, central high fever with restlessness, and delirium [1, 2]. In fact, C+P has shown to reduce the volume of cerebral infarction after ischemia/reperfusion (I/R) injury and improve neurological outcome in an animal study [3]. This study aims to investigate the role of endoplasmic reticulum stress-associated PERK-eIF2α pathway in cerebral I/R injury and the effect of C+P in this pathway. The findings can help understand the neuroprotective mechanisms of C+P and provide additional therapeutic targets.
Methods: A total of 49 male Sprague Dawley rats (280-320g) were randomly divided into 4 different groups (n=7 per group): (1) sham, (2) I/R group that received 2 hours of middle cerebral artery occlusion (MCAO), followed by 6 or 24 hours of reperfusion, (3) 2-hour MCAO treated by C+P without temperature control and (4) 2-hour MCAO treated by C+P with temperature control. SH-SY5Y cells were also divided into 5 groups: (1) control, (2) oxygen–glucose deprivation (OGD) for 2 hours and re-oxygenation (OGD/R), (3) OGD/R with C+P; (4) OGD/R with PERK inhibitor (i.e., GSK2656157), and (5) OGD/R with C+P combined with GSK2656157. Western blotting was used to measure the protein expressions of ERS, UPR (Bip, PERK, p-PERK, p-eIF2α), autophagy (ATG12, LC3Ⅱ/Ⅰ), and apoptosis (BAX, Bcl-XL). mRNA expression of these molecules was detected by RT-PCR.
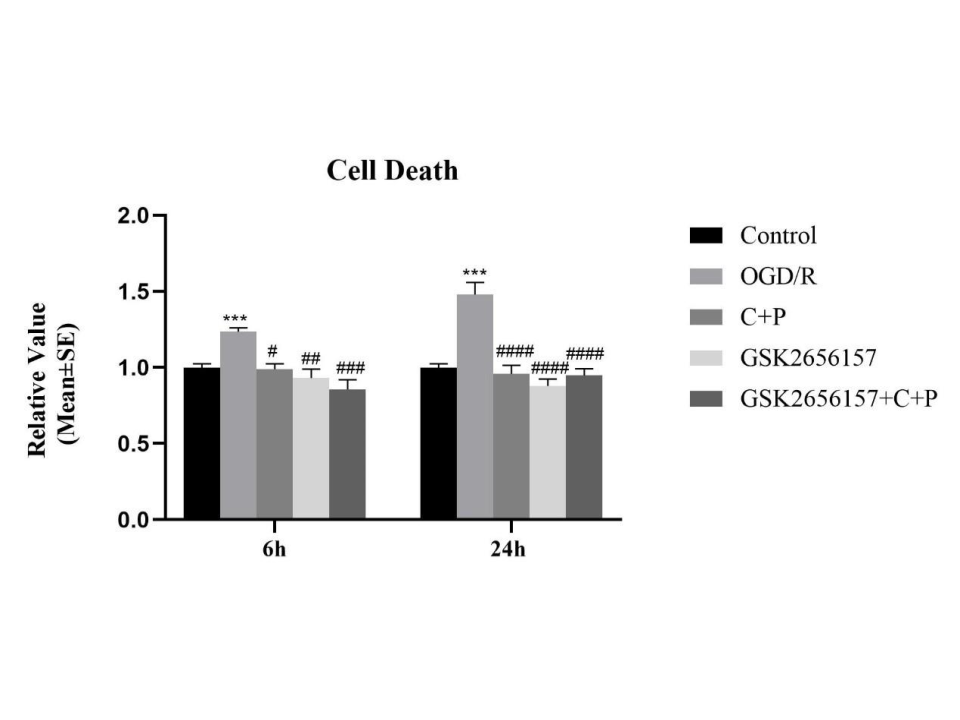
Results: This animal MCAO-model study showed increased expression of PERK-eIF2α pathway related proteins, including Bip, p-PERK, PERK, p-eIF2α, ATG12, and LC3Ⅱ/Ⅰ after I/R. These markers were significantly reduced by C+P administration. In both treatment groups, anti-apoptotic Bcl-XL protein expression was increased while pro-apoptotic BAX proteins was decreased. In SH-SY5Y cell lines, the application of PERK specific inhibitors (i.e., GSK2656157) and C+P significantly reduced the level of autophagy and apoptosis after I/R. However, the combination of GSK2656157 and C+P did not promote the same effect.
Conclusions: C+P inhibits endoplasmic reticulum stress-mediated autophagy activation after cerebral I/R, reduces cell apoptosis and exerts neuroprotective effects. Reduction of body temperature either minimally or did not enhance “C+P-induced neuroprotection.” The PERK-eIF2α pathway is a potential target for C+P to exert its neuroprotective effects.