Background: Clostroidiodes difficile (CD) is the most prevalent hospital-acquired infection in the United States, accounting for approximately 224,000 infections with 13,000 deaths and over 1 billion dollars spent in 2017, according to the Centers for Disease Control and Prevention (CDC). Clostroidiodes difficile accounts for 10-20% of diarrhea in the setting of recent antibiotics exposure.In St – Francis- Emory Healthcare we started and implemented five interventions after doing a Gap analysis to reduce the incidence of hospital-acquired Clostridium difficile infection (HA-CDI) resulting in zero HA- CDI cases from June 2021 to June 2022.
Purpose: We conducted a retrospective study to assess the effectiveness of interventions implemented to reduce the rate of HA-CDI at St Francis – Emory Healthcare (SFEF) from June 2021 to the 2022 year to date (YTD – June 24, 2022). Interventions included: proton pump inhibitor stewardship, antimicrobial stewardship, hand-hygiene accountability, a diarrhea decision tree, and an updated CDI testing algorithm.
Description: This project was conducted between June 2021 and June 2022 at St Francis- Emory Healthcare hospital a 325-bed community hospital in Columbus, Georgia. An interprofessional CDI (Clostroidiodes difficile infection) Prevention team was formed, including representatives from quality improvement, administration, IT, nursing, laboratory department, and a physician champion, in April 2021 to discuss ways to reduce HA-CDI within the hospital. The team conducted a gap analysis based on multidisciplinary experience to determine what needed to be implemented. Ultimately interventions included: an antimicrobial stewardship program, a diarrhea decision tree, a proton pump inhibitor use algorithm, an adjustment of the CDI testing protocol, and a handwashing surveillance program. The HA-CDI rates were reduced significantly after these interventions with improved patient care and saved the hospital hundreds of thousands of dollars.
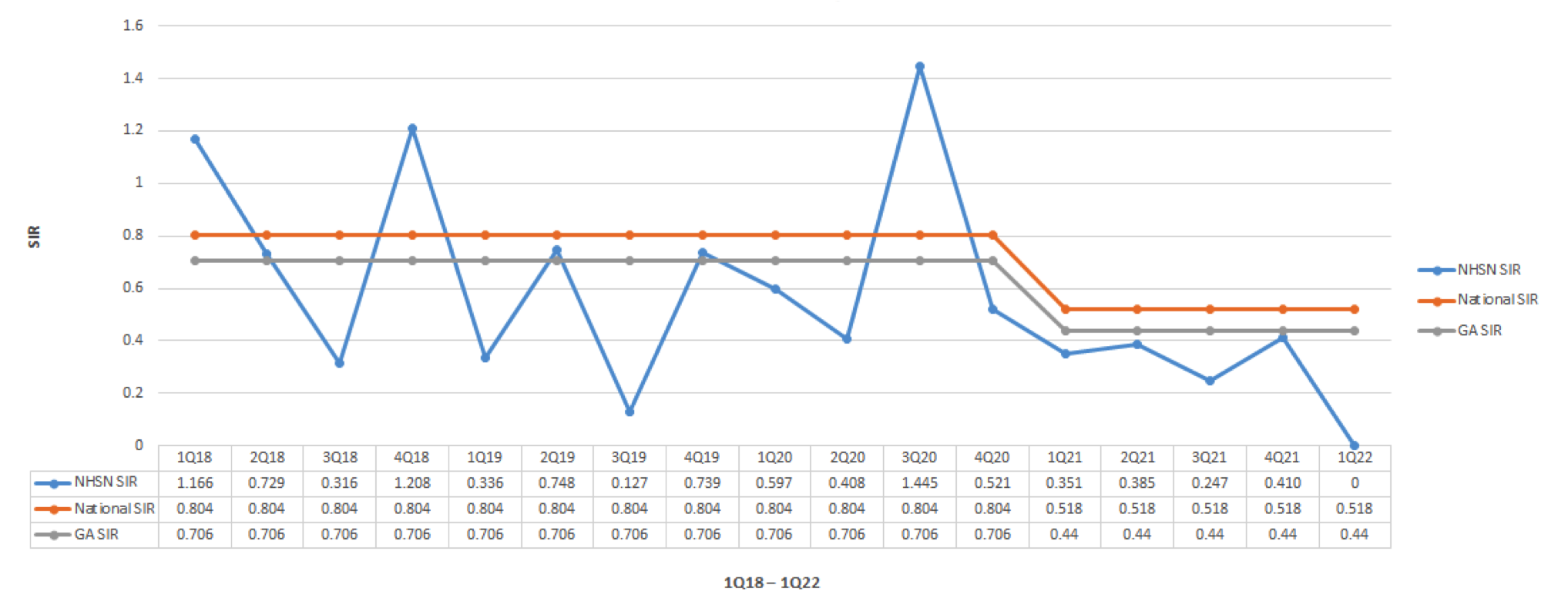
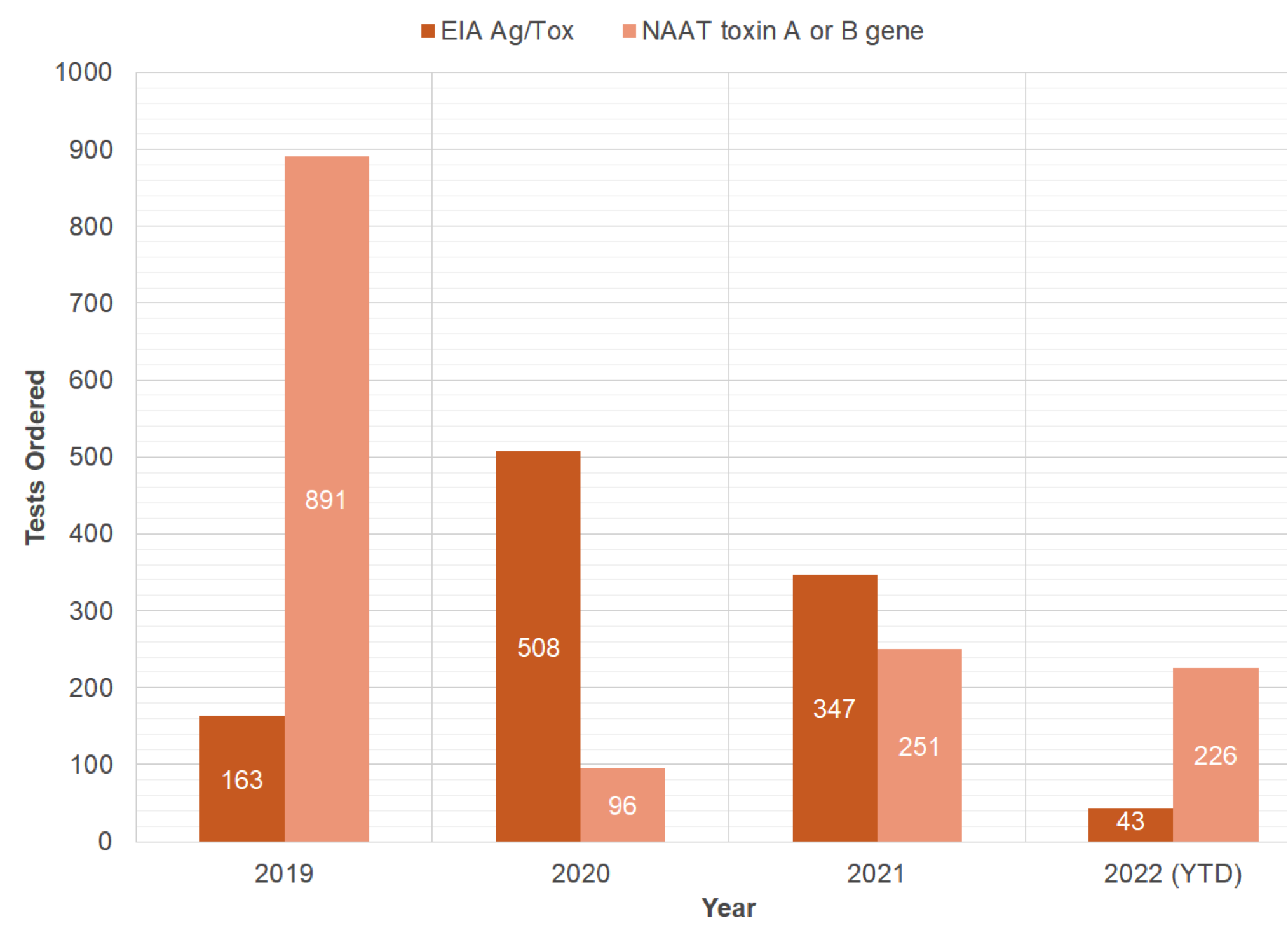
Conclusions: There have been 0 hospital-acquired clostridioides difficle at Saint Francis – Emory Healthcare (SFEH) since implementing interventions starting in June 2021. The diarrhea decision tree was implemented in the EMR which resulted in less total laboratory testing because it removed the testing samples that did not fit the criteria for testing. Previously the EIA Ag/Toxin A or B has periodically been used as a screening test leading to false positives, but this was corrected with the NAAT/PCR which has a specificity of ≥ 97% and sensitivity of≥ 90%. The decrease in HA-CDI can mainly be attributed to the antimicrobial stewardship program limiting specific antibiotic use, using the NAAT/PCR for screening, using the diarrhea decision tree for testing, proton pump inhibitor algorithm, and promoting hand hygiene. In our experience to reduce the Hospital Acquired Clostroidiodes difficile best results are achieved by an interdisciplinary approach.