Case Presentation:
A 21‐year old man had been admitted to the ICU for a 30% burn injury incurred in a house fire and explosion. He underwent excision and skin grafting and was receiving 60‐100 mg of morphine a day via continuous infusion and intermittent boluses. The patient was extubated but was unable to effectively feed enterally because of high gastric residuals and no bowel sounds over 4 days. An average enteral feed of 1200 cc/day resulted in gastric residuals of 750 cc/day. With approval from the University of Chicago institutional review board, the patient received 8 doses of methylnaltrexone (MNTX), 0.3 mg/kg, infused over 10 minutes, every 6 hours over 2 days. He continued receiving morphine via bolus and continuous infusion.
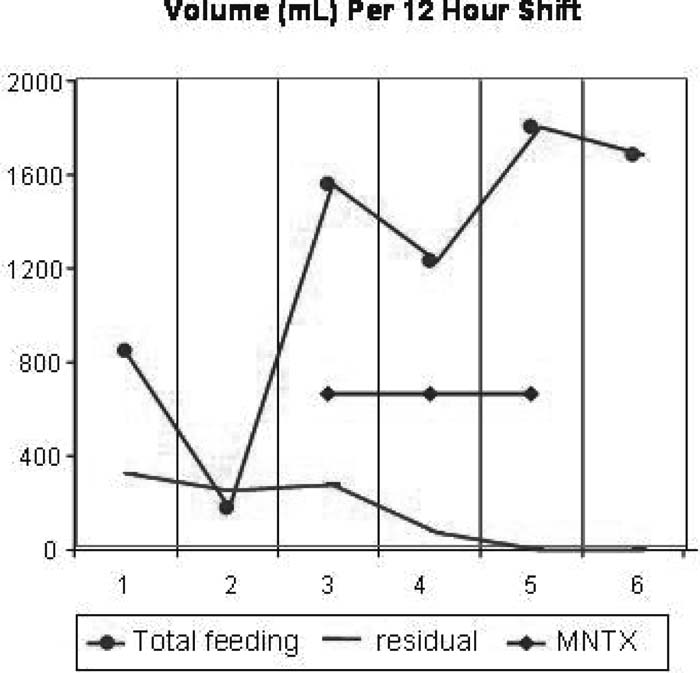
The MNTX was well tolerated and did not elicit pain or withdrawal symptoms at any time. Flatus occurred immediately during the first dose of MNTX. Over the subsequent 48 hours, gastric residuals decreased to 0 cc/day. The patient was able to tolerate enteral feeds and averaged more than 2400 cc/day during this time (see figure, right). By day 5 he was tolerating solid food by mouth.
Discussion:
MNTX is a peripheral opioid receptor antagonist that does not cross the blood‐brain barrier in humans. In phase 2 and phase 3 clinical trials subcutaneous MNTX reversed opioid‐induced bowel dysfunction in patients with advanced illness without reversal of analgesia or withdrawal. In a phase 2 postoperative bowel dysfunction study of the IV formulation, the drug accelerated bowel recovery by 20 hours without reversal of analgesia or withdrawal. MNTX has also been demonstrated to reverse opioid‐induced gastric retention in normal subjects. We hypothesized that MNTX might benefit critically ill patients with significant gastric dysfunction by improving enteral feeding.
This report describes the marked effect of MNTX on gastric dysfunction in a critically ill burn patient. The results suggest a reversal of an undesired peripheral opioid effect (delayed gastric emptying) without apparent diminution of analgesia.
Conclusions:
MNTX was well tolerated and resulted in immediate improvement in feeding and gastric residuals in this critically ill patient. This experience suggests a potential role for methylnaltrexone in the critical care setting.
Author Disclosure:
J. Moss, Progenics Pharmaceuticals, Inc., stock options or bond holdings, consulting fees or other remuneration (payment); M. C. Woo, None; C. S. Yuan, Progenics Pharmaceuticals, Inc., consulting fees or other remuneration (payment); R. J.Israel, Progenics Pharmaceuticals, Inc., stock options or bond holdings, employment (full‐ or part‐time).