Background: Sodium Polystyrene Sulfonate (SPS, Kayexalate) has commonly been prescribed for patients with hyperkalemia since 1958. However, reports of severe gastrointestinal side effects, particularly intestinal necrosis, have been persistent since the 1970s, leading some authors to recommend newer costlier alternatives to SPS. A prior systematic review of the literature found 30 separate case reports or case series including a total of 58 patients who were treated with SPS and developed severe gastrointestinal side effects. No prior systematic review has included controlled studies that report the rate of intestinal necrosis associated with SPS compared to controls.
Methods: A systematic literature search of published studies was conducted using Cochrane Library, Ovid Embase, Ovid Medline, Google Scholar, Pubmed, Scopus, and Web of Science Core Collection from database inception through October 4, 2020. We included any clinical trial, cohort or case-control study that reported an association between SPS receipt and intestinal necrosis or severe gastrointestinal side effects in adults. Two reviewers independently performed data extraction and quality assessment using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool and the Cochrane risk of bias tool. The risk of intestinal necrosis and the composite outcome of severe gastrointestinal disease associated with SPS were pooled using random-effects meta-analysis. Heterogeneity was estimated using the I² statistic and overall strength of evidence was assessed using GRADE.
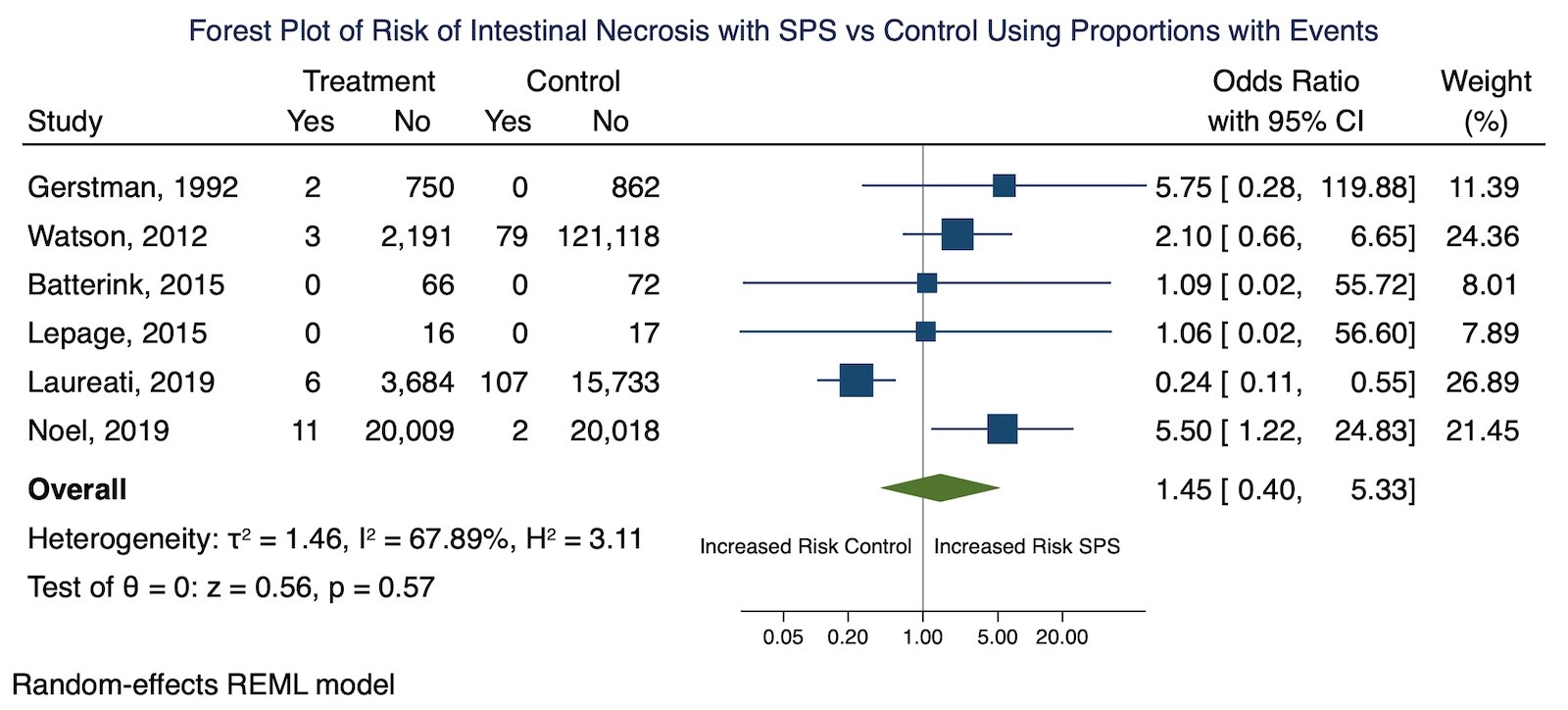
Results: 806 studies were screened for inclusion. Six studies including 26,716 patients treated with SPS with controls met inclusion criteria. The pooled odds ratio of intestinal necrosis from the six studies that reported the proportion of patients that were exposed to SPS that developed intestinal necrosis was 1.45 (95% CI 0.40-5.33). The pooled hazard ratio for intestinal necrosis from the two studies that performed survival analysis was 2.00 (95% CI 0.45-8.78). The pooled hazard ratio for the composite outcome of severe gastrointestinal adverse events was 1.46 (95% CI 1.01-2.11). Most studies had high risk of bias. Overall strength of evidence was low.
Conclusions: Based on our review of six controlled studies, the risk of intestinal necrosis with SPS is small and not statistically greater than controls although there was a statistically significant increase risk for the composite outcome of severe gastrointestinal side effects based on two studies. Due to risk of bias from potential confounding and selective reporting, the overall strength of evidence to support an association between SPS and intestinal necrosis or other severe gastrointestinal side effects is low. The use of costlier new medications from the point of safety is not currently supported by the published literature.