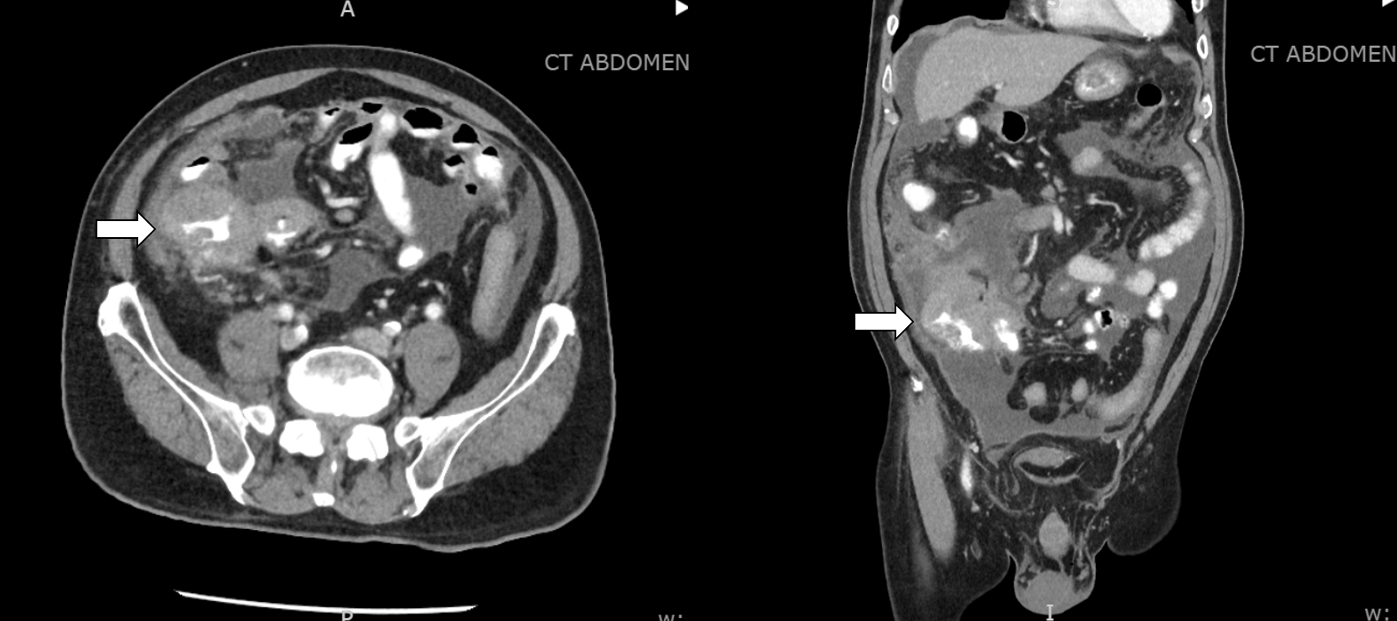
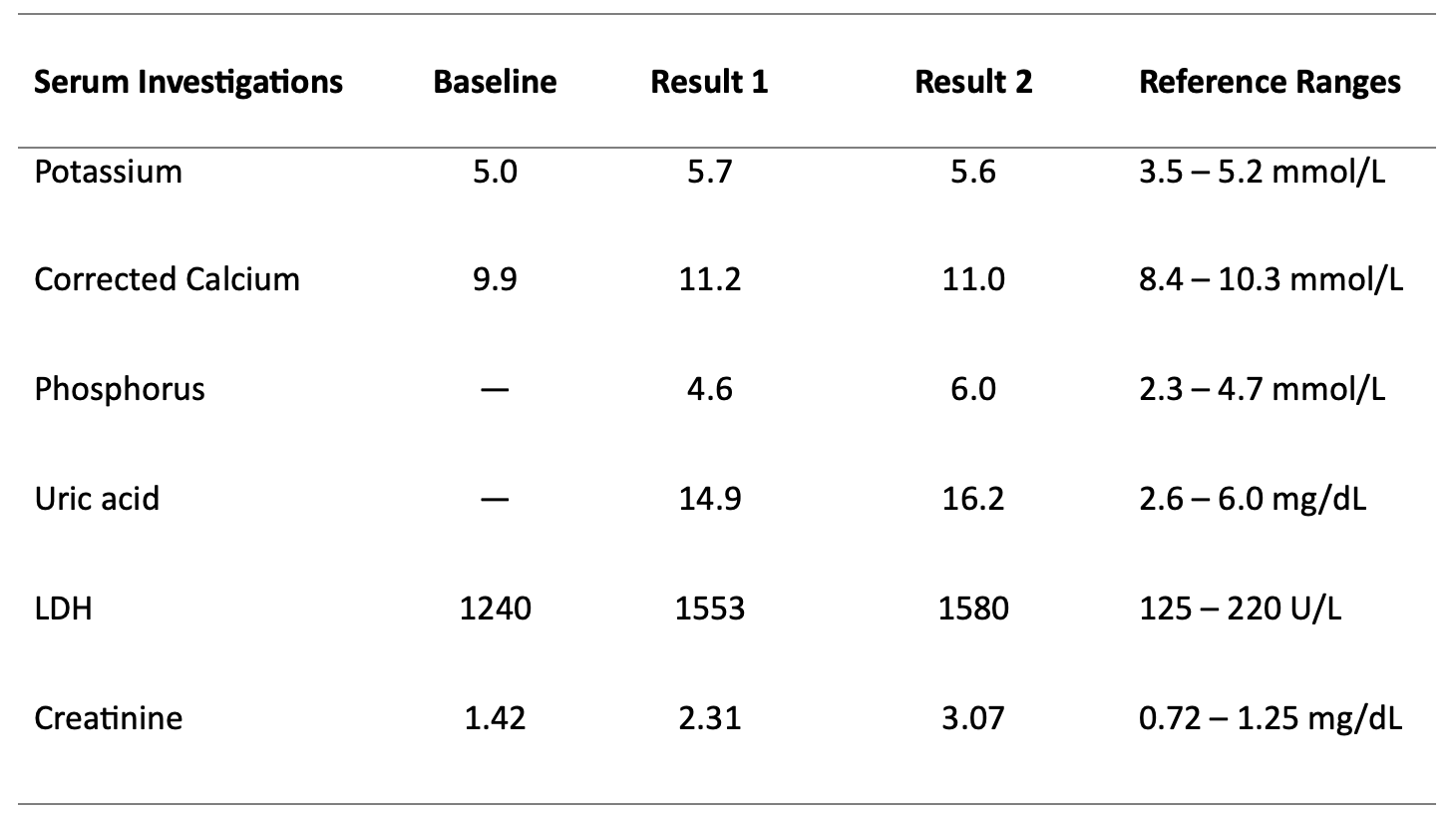
Case Presentation: An 81-year-old man with a history of heart failure (HF) and recent diagnosis of an ileocecal mass with associated bulky lymphadenopathy and peritoneal carcinomatosis (Figure 1) presented to the emergency room with decreased appetite, abdominal pain, and worsening abdominal distention. Upon arrival, the patient was afebrile and hemodynamically stable. Physical examination was remarkable for a distended abdomen with a positive fluid wave. Blood investigations revealed acute kidney injury (AKI), hyperkalemia, hypercalcemia, high-normal phosphorus levels, and increased lactate dehydrogenase (Table 1). Initially, AKI from decreased intake was suspected, but due to the concern for tumor lysis syndrome (TLS), a uric acid level was checked and found to be elevated at 14.9mg/dL (reference range: 2.6 – 6.0mg/dL). The patient was started on intravenous fluids and rasburicase. Allopurinol was also added due to rising uric acid levels. Subsequent ascitic fluid cytology and immunohistochemical analysis confirmed a diagnosis of diffuse large B-cell lymphoma (DLBCL), non-germinal center B-cell-like subtype, providing further clarity to the diagnosis of spontaneous TLS.
Discussion: TLS is a critical oncometabolic emergency, typically seen in patients undergoing cytotoxic therapy or radiotherapy for malignancies with a high tumor burden. The Cairo-Bishop TLS laboratory criteria involves hyperuricemia, hyperkalemia, hyperphosphatemia, hypocalcemia, and uremia [1]. This results from the breakdown of a large number of malignant cells that release intracellular ions and nucleic acids into the systemic circulation. With an incidence as low as 1.8%, spontaneous TLS is a rare entity, especially with solid organ malignancies, and patients often experience higher mortality rates [2,3]. Our case is unique as the patient presented with a newly discovered solid tumor pending a definitive cancer diagnosis. Additionally, at initial presentation, the patient exhibited atypical electrolyte findings with hypercalcemia, which could be from the underlying DLBCL, and a high-normal phosphorus level. Furthermore, in the setting of HF and decreased oral intake, there are more common differentials of AKI that may complicate the diagnostic process. In this case, we treated the patient empirically. The dynamic changes in the laboratory results and subsequent confirmation of underlying DLBCL made TLS a more certain diagnosis.Timely identification of TLS is crucial because the early initiation of medical treatment aimed at correcting the metabolic derangements reduces the likelihood of harmful complications such as cardiac arrhythmia, renal failure, seizures, and even death [3,4]. The renal dysfunction observed in TLS typically ensues from intrarenal precipitation of calcium phosphate salts and uric acid. Rasburicase, a recombinant urate oxidase, is the preferred agent over allopurinol for lowering uric acid levels. Renal replacement therapy is indicated for renal failure and electrolyte derangements that are refractory to medical therapy [3,4].
Conclusions: Spontaneous TLS should be considered foremost among the differential diagnoses in patients with a known or possible underlying malignancy, regardless of suspected tumor type, who present with AKI and suspicious metabolic derangements without exposure to cancer therapy. If TLS is highly suspected, prompt management of electrolyte disturbances, hyperuricemia, and renal failure is essential for prevention of life-threatening complications.