Background:
Subcutaneous (SC) administration of human recombinant hyaluronidase (rHuPH20) temporarily increases connective tissue permeability by degrading hyaluronan. Because this technique enhances dispersion of coadministered molecules, we evaluated whether rHuPH20 coadministered with SC morphine enhances morphine absorption.
Methods:
Two placebo‐controlled, double‐blind, crossover trials, one with 13 palliative care patients and the other with 18 healthy volunteers, randomized individuals to a sequence of SC morphine + saline, SC morphine + rHuPH20 (150 units), and unblinded IV morphine on consecutive days. Morphine doses were 2 mg (healthy volunteers) and 5 mg (palliative care). Plasma levels of morphine and its active metabolite morphine‐6‐glucuronide (M6G) were determined at baseline and defined intervals. The primary end point was comparative evaluation of time to maximum morphine plasma concentration (Tmax); other pharmacokinetic (PK) end points were maximum plasma concentration (Cmax) and area under the plasma concentration‐time curve (AUC). Safety and tolerability were also assessed.
Results:
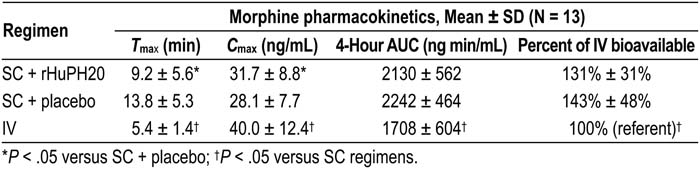
In palliative care patients (see table), SC morphine + rHuPH20 significantly enhanced morphine absorption relative to SC + placebo, resulting in a 33% shorter Tmax and a 13% higher Cmax (both P < .05). AUC and bioavailability with SC administration were no less than IV. Data from the volunteer study (based on inter‐subject comparison from day 3; n = 6 for each regimen) were consistent with the palliative care population; relative to placebo, concurrent administration of rHuPH20 reduced morphine Tmax by 58% (P < .05) and increased Cmax. Also similar to the palliative care study, volunteers had AUC with SC administration with and without rHuPH20 that was no less than IV. In both populations, PK of M6G after SC morphine was comparable to IV morphine. All injections were well tolerated. No serious or severe adverse events (AEs) were reported. Transient and generally mild injection‐site reactions were the most common AEs, and their incidence was similar for SC injections in both studies, with the possible exception of pruritus (17% vs. 17% in the patients and 17% vs. 0% in the volunteers for rHuPH20 and saline, respectively).
Conclusions:
rHuPH20 significantly enhanced SC absorption of coadministered morphine in palliative care patients and volunteers and was well tolerated. These data suggest that analgesia may be rapidly achieved using rHuPH20 in patients for whom IV morphine is not feasible. Clinical efficacy of this regimen warrants further evaluation.
Author Disclosure:
J. Thomas, None; R. Yocum, Halozyme Therapeutics Inc., employment (full‐ or part‐time); M. Haller, Halozyme Therapeutics, Inc., employment (full‐ or part‐time); J. Flament, Baxter, employment (full‐ or part‐time); C. Von Gunten, None.