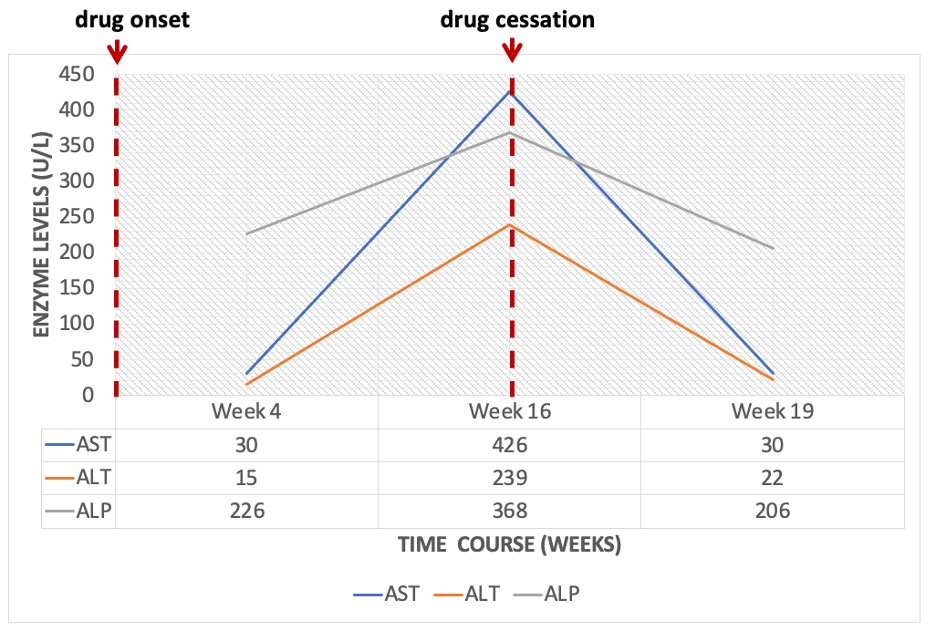
Case Presentation: Diclofenac is a routinely prescribed non-steroidal anti-inflammatory agent in hospital with well-recognized hepatotoxic potential. However, there are only two reported cases of topical diclofenac-induced liver injury in literature. Herein, we describe a case of a 94-year-old man with Paget’s disease and osteoarthritis, who developed acute hepatotoxicity after four months of application of topical diclofenac 1% gel in escalating doses (50g daily as compared to the recommended 32g). Laboratory diagnostics revealed an elevated total bilirubin (4.9 mg/dL (reference range 0.2-0.9 mg/dL)) that was predominantly direct (3.84 mg/dL (reference range 0.00-0.3 mg/dL)), aspartate aminotransferase (426 U/L (reference range 0-33 U/L)), alanine aminotransferase (239 U/L (reference range 10-49 U/L)), and alkaline phosphatase (368 U/L (reference range 46-116 U/L)). The R factor was noted to be 3.2, suggestive of a mixed pattern of liver injury. Given his underlying Paget’s disease and baseline alkaline phosphatase elevation however, a true hepatocellular injury is probable. Importantly, AST and ALT enzymes as well as total bilirubin were within the normal ranges about three months prior to presentation. Once diclofenac gel was discontinued, repeat blood work three weeks after discharge demonstrated return of his liver function tests to baseline.
Discussion: The incidence of diclofenac-induced symptomatic liver disease requiring hospitalization is estimated to be around 23 persons per 100,000 exposed. Initial presentations with jaundice and elevated serum aminotransferases (>3 times the upper limit of normal), have been associated with 14% risk of mortality secondary to acute liver failure. Given the temporal relationship between the bioavailability of topical diclofenac and the changes in the liver function tests in this patient, the likelihood of diclofenac-induced liver injury was deemed possible using an internationally recognized causality assessment tool. While the patient was on other potentially hepatotoxic medications, topical diclofenac gel was the only relatively new medication and was misused. Furthermore, the viral panel was negative, etiologies for obstructive cholestasis were ruled out on CT imaging, and suspicion for autoimmune hepatitis was low.
Conclusions: In most cases, patients with diclofenac-associated liver injury are asymptomatic and achieve complete hepatic recovery within 4-6 weeks of drug withdrawal. Nevertheless, rare cases of chronic liver injury necessitating corticosteroid treatment have been reported. Thus, further research on topically administered non-steroidal anti-inflammatory agents is needed to identify routine monitoring intervals for early detection and avoidance of adverse effects.