Case Presentation:
A 53 year-old woman with history of depression and chronic kidney disease stage 2 – with baseline serum creatinine (Cr) of 1 mg/dl – presented with a Cr of 2.9 mg/dl obtained as part of preoperative work up for spinal stenosis. She had unintentionally lost 7 pounds over 3 months. She had no history of exposure to herbal drugs, recent NSAIDS or IV contrast use. She had been on topiramate 100 mg twice daily for 38 months for depression. Physical exam was completely normal.
Upon review of her lab work (table), she was found to have a hyperchloremic non-gap acidosis with normal serum glucose. She also had low serum uric acid and hypokalemia. Urine electrolytes revealed an anion gap of 30.7 and a fractional excretion of sodium (FENa) of 3. Urine analysis showed proteinuria and glucosuria with no evidence of infection; it had been normal nine months before.
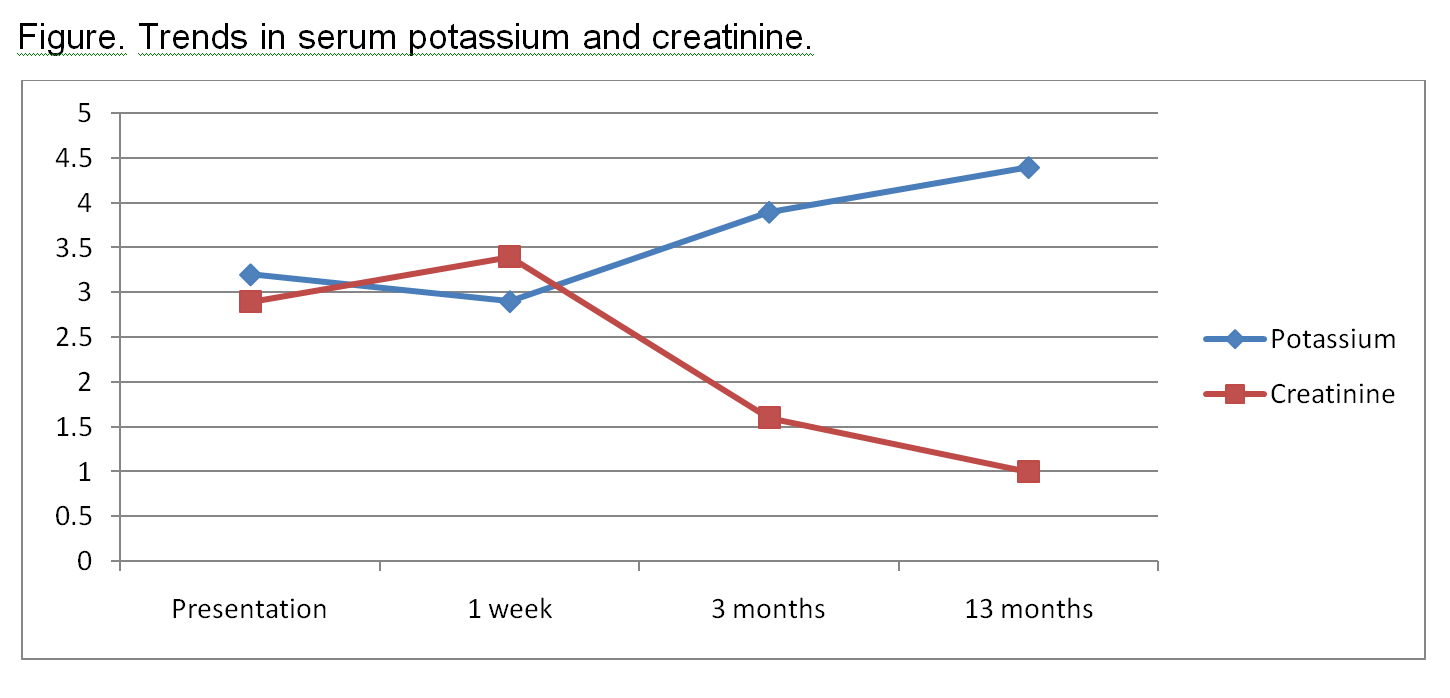
The foregoing suggested proximal tubular dysfunction. Given her elevated creatinine, proteinuria, and anemia, a paraproteinemia was strongly considered. So serum/urine protein electrophoresis and free Kappa/ Lambda ratio were sent which were negative. There was yet high suspicion for multiple myeloma, so the patient underwent a bone marrow biopsy that was negative. Thus proximal renal tubular acidosis (RTA) with acute kidney injury (AKI) secondary to topiramate (TPM) was entertained. The Naranjo adverse drug reaction probability scale indicated a probable relationship (score of 6) between TPM and AKI and RTA, so TPM was withdrawn. Few days after tapering started; there was improvement of acidosis and parameters of kidney function until she had normal serum bicarbonate and uric acid. These changes occurred over a few weeks and were accompanied by gradual improvement of her Cr that returned to baseline by 13 months after cessation of TPM (Table and Figure).
Discussion:
Topiramate use has been associated with non anion gap metabolic acidosis without regard to dose through inhibition of type II and IV carbonic anhydrase though it is rare in adults. Metabolic acidosis due to topiramate therapy has not typically been associated with creatinine elevation. Only one case of probable topiramate-induced acute renal failure has been reported; renal failure was severe enough to warrant hemodialysis. Our case is unique as TPM not only caused normal anion gap metabolic acidosis years after initiation but was also associated with AKI that resolved with discontinuation of the drug. To our knowledge this is the first report of such a scenario. Recognition of drug-induced electrolyte imbalance and/or renal dysfunction is crucial so that the drug can be withdrawn while supportive care is provided.
Conclusions:
Considering that the onset of metabolic acidosis during topiramate therapy appears unpredictable, we recommend measuring serum bicarbonate, potassium and creatinine before initiation of topiramate therapy and at regular intervals when this drug is being administered to any patient. Further research into the most effective scheme of monitoring kidney function and electrolytes while on topiramate therapy is required.
Table. Serial serum chemistries.
|
|
At time of initial evaluation |
1 week after (Topiramate was withdrawn after this test) |
3 months after stopping drug |
13 months after stopping drug |
|
Sodium |
142 |
138 |
139 |
138 |
|
Potassium |
3.2 |
2.9 |
3.9 |
4.4 |
|
Chloride |
114 |
106 |
102 |
100 |
|
Bicarbonate |
18 |
14 |
26 |
30 |
|
BUN |
17 |
21 |
15 |
24 |
|
Creatinine |
2.9 |
3.4 |
1.6 |
1 |
|
Glucose |
110 |
131 |
118 |
104 |
|
Calcium |
9.2 |
9.1 |
9.2 |
9.2 |
|
Phosphorus |
3.9 |
2.9 |
3.7 |
3.6 |
|
Uric acid |
1.9 |
2.8 |
2.3 |
3.7 |
|
Total protein |
7.9 |
7.9 |
7.3 |
7.2 |
|
Albumin |
4.1 |
4.1 |
3.9 |
3.9 |