Background: Panel-based Nucleic acid amplification tests (NAATs) are laboratory tools that are used to detect small quantities of RNA or DNA to rapidly identify a multitude of infectious organisms. A systematic review and meta-analysis looking at the diagnostic accuracy of the BioFire® FilmArray® meningitis-encephalitis (ME) panel which is used at our institution showed that it has a sensitivity and specificity of 90% and 97% respectively. At our institution, a 700-bed tertiary referral county hospital, these ME panels are part of the lumbar puncture order set and therefore are ordered prior to knowing the results of the CSF analysis. The cost for the ME panel is between $750 – 950. We sought to investigate the impact of sending the MEP on CSF samples collected from 2017-2020 from both medical decision making and financial perspectives. We hypothesized that in immunocompetent patients, a normal total nucleated count (< 5 cells/mm3) in CSF might be sufficiently sensitive to rule out meningoencephalitis, eliminating the need for the ME panel and thereby reducing overall cost.
Methods: We retrieved a list of all ME panels sent at our institution between 2017 and 2020. A total of 1759 samples were analyzed. We considered the CSF WBC to be positive if any WBC count was ≥5 cells/mm3. Our definition of immunocompromised was if the patient had AIDS, Leukopenia, and/or on immunosuppressant medications. For all specimens with positive ME panel and negative (< 5) CSF WBC, we performed chart reviews to determine if the patient was immunocompromised, and to determine the clinical significance of the ME panel result.
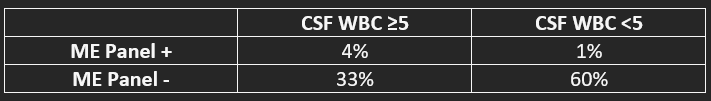
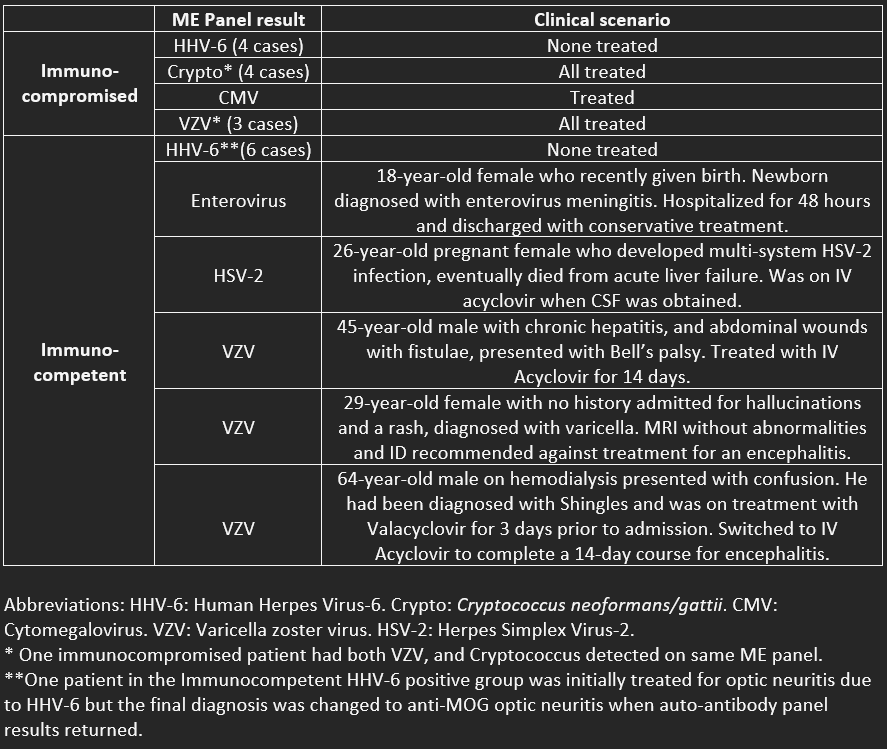
Results: Of 1759 samples, distribution of results are seen in table 1. Out of the 100 specimens with positive ME panels, below we will focus on the 22 which had a CSF WBC of < 5:- None were from bacterial pathogens, and all were in an adult population ≥18 years old. - 11 were from clearly immunocompromised patients (HIV, leukopenia, or on immunosuppressive medications for transplant or autoimmune diseases.) - Among immunocompetent and immunocompromised patients, a total of 10 were positive for HHV-6. None of these were considered significant or require antiviral treatment. - 5 were found to be immunocompetent and have true positive ME panel results in the absence of elevated WBC.
Conclusions: In our cohort of 1759 ME panel results, a strategy to reduce the use of the ME panel by sending the test in immunocompetent patients only when CSF WBC is ≥5 cells/mm3 would have identified 80 out of 85 (95%) positive ME panel results while eliminating 10 false positive HHV-6 results and missing 5 (6%) cases where viral DNA/RNA was present in the CSF, with comments regarding management decisions highlighted in Table 2. False positive results can be due to numerous causes including amplification of a contaminating organism, lack of specificity of the assay primer or probe sequences, detection of RNA/DNA causing an infection outside the central nervous system, or detection of a latent infection such as HHV-6. Although it remains up for debate whether CSF WBC count is sufficiently sensitive to rule out meningoencephalitis in immunocompetent patients, it is likely to be sufficient when pretest probability is low. Through further research, if the CSF WBC count is deemed to be useful as a tool to determine whether a ME panel should be performed, the cost savings would be significant.