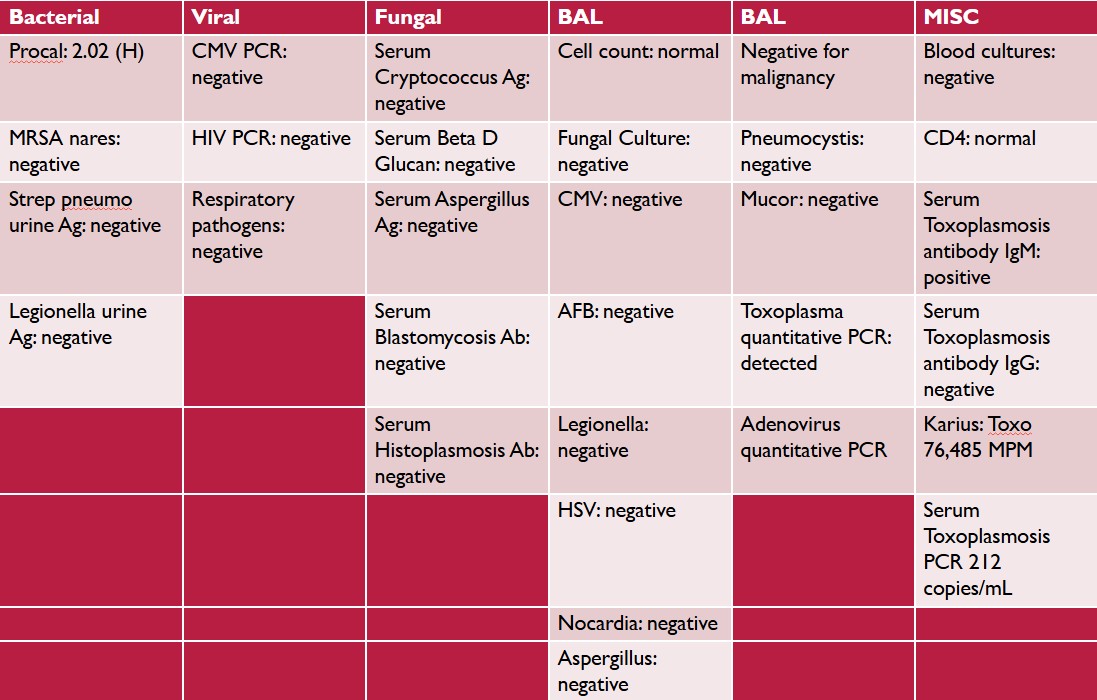
Case Presentation: We present a case of toxoplasmosis pneumonia in a 56-year-old male with a medical history significant for renal transplantation due to familial focal segmental glomerulosclerosis (FSGS) seven years ago. His post-transplant course was complicated by development of chronic kidney disease (CKD), and he was on maintenance immunosuppression. The patient presented to the emergency department with a two-week history of fever, fatigue, decreased oral intake, diaphoresis, and weight loss. He also reported a two-day history of cough and intermittent headaches. On physical examination, the patient was febrile, and his white blood cell count was 3.5 k/uL. Initial lab work revealed acute on chronic kidney injury. A CT scan of the chest showed bilateral patchy coarse reticular and ground-glass opacities (Figure 1), while a CT head was unremarkable. Nasopharyngeal respiratory viral PCR was negative. Despite several days of broad-spectrum antibiotics, the patient’s fever persisted and worsened, and he developed hypoxia, requiring supplemental oxygen, prompting transfer to a tertiary care center for bronchoscopy and further evaluation. An extensive laboratory work-up was negative for bacterial, viral, and fungal pathogens. HIV testing was negative, and his CD4+ T-cell count was 211 cells/mcL. Serum Toxoplasma IgM antibodies were positive, and IgG antibodies were negative. Serum PCR revealed Toxoplasma gondii with 2,500 copies/mL. Further testing with blood microbial cell-free DNA (Karius) identified Toxoplasma gondii with 76,485 molecules per microliter. Bronchoscopy and bronchoalveolar lavage (BAL) demonstrated Toxoplasma pneumonia, with quantitative PCR showing 212 copies/mL. The complete diagnostic work-up is shown in (Figure 2). The patient was diagnosed with acute toxoplasmosis pneumonia and was started on pyrimethamine, sulfadiazine, and leucovorin based on infectious disease recommendations. Mycophenolate was temporarily withheld, while tacrolimus and prednisone were continued as per transplant center guidelines. Magnetic resonance imaging (MRI) of the brain was normal. Due to difficulty obtaining sulfadiazine outpatient and concerns about CKD, the patient was switched to high-dose atovaquone prior to discharge, with a recommended treatment duration of six weeks, followed by lifelong suppression.
Discussion: Toxoplasma gondii is an obligate intracellular protozoan and a leading cause of foodborne illness in the U.S. Transmission occurs through contact with oocysts from infected animals, raw or undercooked meat, or solid organ transplantation. In immunocompetent hosts, infections are usually asymptomatic; however, in immunosuppressed individuals, more severe disease manifestations can occur, including cerebral toxoplasmosis, myocarditis, and chorioretinitis. Pulmonary toxoplasmosis is a rare manifestation. A comprehensive history is key in identifying infection sources. Our patient later reported consuming venison butchered and prepared by a friend about three weeks prior to his presentation, likely the source of infection. Although latent infection from organ transplantation was possible, it was unlikely due to the timing—seven years post-transplant—and the serology pattern indicating an acute infection (IgM positive, IgG negative).
Conclusions: This unique case of toxoplasmosis pneumonia in a non-HIV-infected individual underscores the importance of maintaining a high index of suspicion for rare infections in immunosuppressed patients.