Background: Clostridioides difficile is an opportunistic pathogen that causes an estimated 500,000 infections per year in the United States. RBX2660, a standardized, microbiota-based live biotherapeutic, is being investigated as a treatment option for recurrent C. difficile infection (rCDI). RBX2660 has demonstrated consistent tolerability and efficacy in reducing rCDI across 6 clinical studies. We present a post hoc subgroup analysis on the long-term efficacy and safety of RBX2660 in participants with rCDI in PUNCH CD Open-Label (NCT02589847), a prospective, multicenter, open-label, phase 2 trial.
Methods: PUNCH CD Open-Label participants were enrolled between October 1, 2015, and March 6, 2017, were ≥18 years old, and had either ≥2 episodes of rCDI treated by standard-of-care antibiotic therapy after a primary episode of C. difficile infection (CDI) or at least 2 episodes of severe CDI requiring hospitalization. All participants had a positive stool test (test unspecified) for C. difficile within 60 days before enrollment and were on antibiotics to treat CDI at the time of enrollment. Participants received up to 2 doses of RBX2660 rectally administered 7 ± 2 days apart. Treatment success was defined as the absence of CDI diarrhea without the need for CDI retreatment for 8 weeks after completing study treatment. Treatment-emergent adverse events (TEAEs) were defined as events with onset dates on or after the start of study treatment and were collected through 6 months. Long-term safety data were recorded at 6 and 12 months after treatment, and serious adverse events occurring between 12 and 24 months were recorded during the study exit interview. In this post hoc subgroup analysis, we report the 6-, 12-, and 24-month outcomes of 8-week responders by age, sex, race, and number of prior CDI episodes.
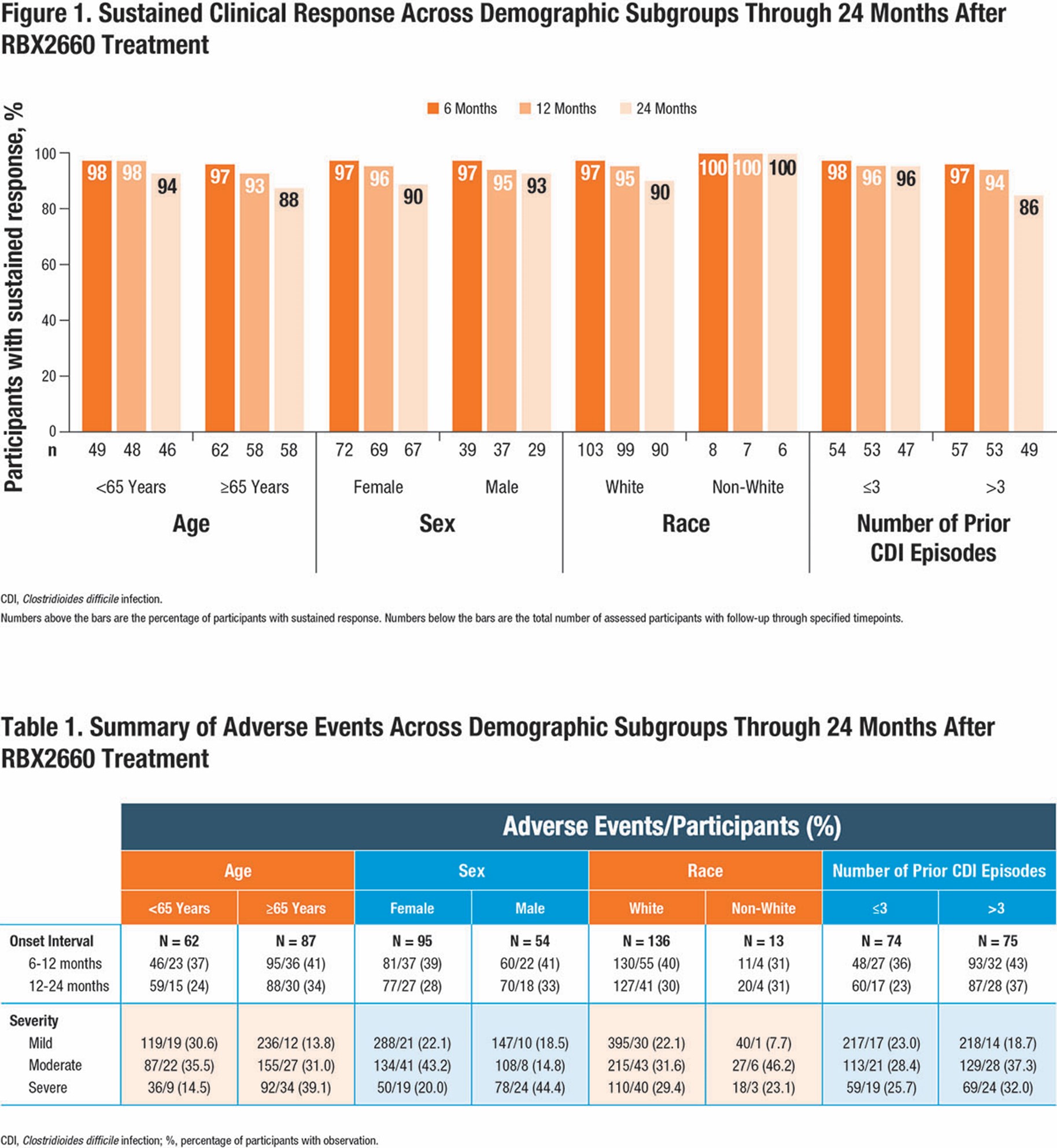
Results: Among the 142 participants treated with RBX2660 (evaluable population), 92% were white, 62% were female, 59% were ≥65 years of age, and 49% had >3 prior episodes of CDI. Sustained treatment response through 6, 12, and 24 months after treatment, respectively, was achieved by 98%, 98%, and 94% of participants < 65 years of age and by 97%, 93%, and 88% of participants ≥65 years of age (Figure 1). Similar sustained response rates were demonstrated in participants when categorized by sex, race, and number of prior CDI episodes. As shown in Table 1, TEAEs were reported by a similar percentage of participants across demographic subgroups between 6 and 12 months (range, 31% to 43%) and 12 and 24 months (range, 23% to 37%) after treatment with RBX2660, with most being gastrointestinal and mild to moderate in severity.
Conclusions: Across demographic subgroups, the majority of RBX2660 responders showed a sustained clinical response, remaining free of CDI recurrence up to 24 months after treatment. Long-term safety data reinforce that RBX2660 is well tolerated.