Case Presentation: A 55 year-old woman with past history of antiphospholipid syndrome complicated by multiple deep vein thromboses and miscarriages, prior hemorrhagic stroke, chronic slow GI bleed with iron deficiency anemia, thrombocytopenia, and CKD stage 3 presents to the ED with two-month history of progressively worsening shortness of breath and chest discomfort with exertion, which acutely worsened over the last day. Initial evaluation revealed hemoglobin of 3.9 grams/dl and presence of heme-positive stools. Patient denied abdominal pain, hematochezia, or maroon-colored stools. She endorsed black tarry stools which she attributed to her oral iron supplementation.
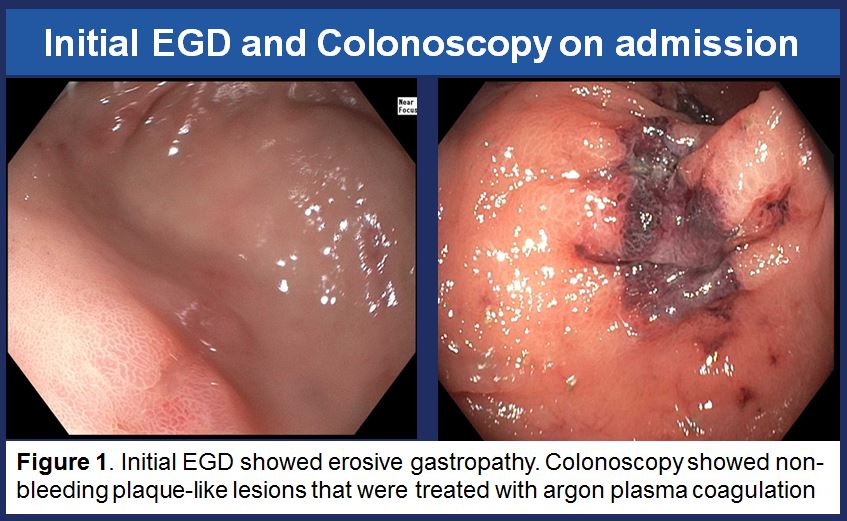
She was admitted to the hospital and received 5 units of packed red blood cells with increase of Hgb to 9.7. Anticoagulation was held. EGD showed erosive gastropathy and colonoscopy showed non-bleeding plaque type lesions which were treated with argon plasma coagulation. Capsule endoscopy and Meckel’s scan were unremarkable. When patient was trialed back on anticoagulation, her Hgb dropped to 7.4.
Her hospitalization was further complicated by AKI on CKD with a peak creatinine of 2.4 and nephrotic range 24-hour proteinuria of 4.5 grams without casts. Serologic work up included positive ANA 1:160 in a speckled pattern, rheumatoid factor, and low C3 and C4. Antiphospholipid antibodies were positive. Other vasculitis and rheumatologic laboratories were negative.
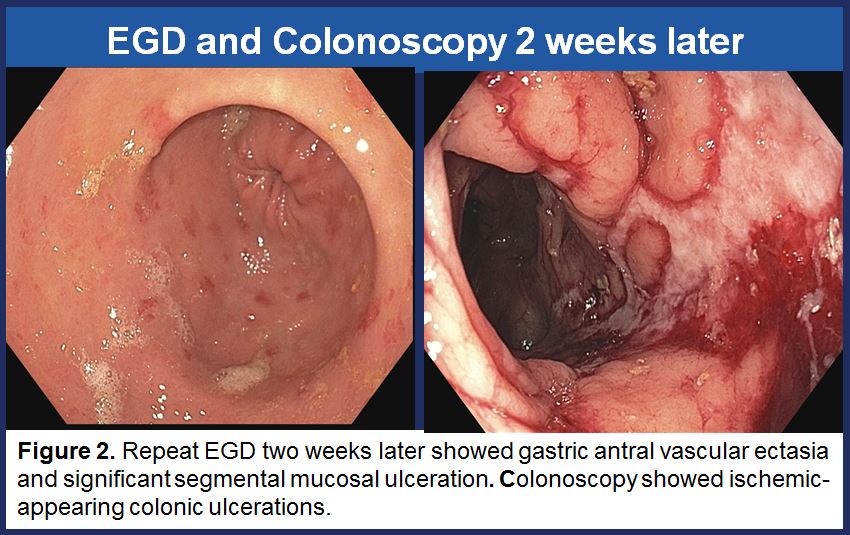
Kidney biopsy showed acute renal microangiopathy with severe tubular atrophy and interstitial fibrosis. Severe hypertensive arteriosclerosis and acute tubular injury was also present. Complete repeat endoscopic work-up showed gastric antral vascular ectasia and significant segmental mucosal ulceration suggestive of ischemia. CT chest showed a wedge-shaped area of infarct in the right middle lobe.
Given her history of APS, the microangiopathy seen on renal biopsy, wedge-shaped pulmonary infarct, and ischemic-appearing colonic ulcerations, it was felt that patient’s overall presentation and severe GI bleed was due to ischemic colitis from micro-infarcts and antiphospholipid syndrome. Patient received a course of IV methylprednisolone, plasmapheresis, and rituximab infusion. She was bridged to a therapeutic level of warfarin with stable hemoglobin prior to discharge from the hospital.
Discussion: Abdominal manifestations of APS are uncommon but can be life threatening. Reported cases of thromboses include Budd-Chiari syndrome, hepatic-veno-occlusive disease and occlusion of small hepatic veins, or hepatic infarction. Intestinal manifestations are even more infrequent. This patient had catastrophic antiphospholipid syndrome defined by 1) Widespread thrombosis in multiple organ systems, 2) development of manifestations simultaneously within a week, 3) confirmation of small-vessel occlusion on histopathology, and 4) laboratory confirmation of antiphospholipid antibodies. This case highlights that abdominal involvement should be considered in patients with APS.
Conclusions: 1. For patients with acute gastrointestinal bleeding and other systemic involvement (in this case, AKI with proteinuria), differential should be broadened to systemic processes.
2. Ischemic colitis should be considered as a cause of GI bleeding in patients with APS
3. Patients with APS-related gastrointestinal bleeding should be discharged on a safe anticoagulation and monitoring plan.