Background: CD-19 Chimeric Antigen Receptor – T (CAR-T) cell therapy is FDA approved for the treatment of primary refractory or recurrent Diffuse Large B-Cell Lymphoma (DLBCL) after two or more lines of systemic therapies have failed. The two commercially available products are Yescarta (axicabtagene ciloleucel) and Kymriah (tisagenlecleucel). In clinical trials, 54% of 111 patients treated with axicabtagene ciloleucel (axi-cel) and 40% of 93 patients treated with tisagenlecleucel with DLBCL had complete responses (CR). The most concerning side effects of CAR-T cell therapy are cytokine release syndrome (CRS) and immune effector cell mediated neurotoxicity syndrome (ICANS).
Methods: We retrospectively reviewed the first ten patients with DLBCL treated with axi-cel between June 2018 and June 2019 at an inner-city tertiary care center post-FDA approval. We determined the rate of clinical remission based on post-CAR-T cell infusion imaging (whole body PET/CT scan). Demographic characteristics and clinical parameters were collected via chart review. HIPAA consent was waived as this was a retrospective study. All patients received lymphodepleting chemotherapy per product guidelines with fludarabine and cyclophosphamide followed by CART cell infusion. Growth factor support and antimicrobial prophylaxis was administered per guidelines.
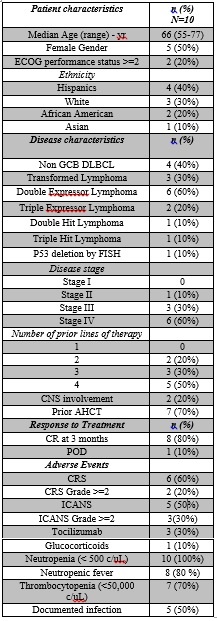
Results: Patient characteristics are shown in Table 1. All ten patients were demographically diverse and had heavily pretreated recurrent or refractory DLBCL. Outcomes were shown on a swimmer’s plot. The median age at the time of CAR-T cell infusion was 66 years (range 54-77). Two patients had prior CNS involvement.Figure 1 shows the follow-up for all patients treated with axi-cel. Out of ten patients, 80% achieved a CR including the patients with prior CNS involvement. One patient had progression of disease within a month of treatment and died 4 months later due to complications of the disease. The other patient developed severe neurotoxicity, required tocilizumab and glucocorticoids, and expired during initial hospital stay.The most common side effect after CAR-T cell infusion was neutropenic fever which occurred in 8 patients (80%) and required treatment with empiric antibiotics. CRS was seen in 6 patients. ICANS was observed in 5 patients. Three patients, who achieved a CR after axi-cel, did not develop CRS or ICANS.
Conclusions: This data supports the feasibility of CAR-T cell treatment to heavily pre-treated patients within a reasonable time frame; roughly one month from collection to infusion and 2-3 weeks of hospitalization during treatment. Providers can expect high rates of remission as well as high frequencies of CRS and ICANS though our series show severe episodes are rare occurring in less than 25% of patients. The complete responses seen in patients treated, especially in patients with concomitant CNS involvement are encouraging for the wider application. The two deaths seen in this series were related to lymphoma progression in onw patient and severe CRS and ICANS in another patient. Our study adds to the evidence of the safety and efficacy of CD-19 directed CAR-T cell therapy in patients with aggressive DLBCL. This series highlights a CR rate higher than that observed in clinical trials in patients with significant comorbidities including CNS involvement. As the experience with administering CAR-T cell therapies outside of a clinical trials continues to grow, it is encouraging to find that patients are tolerating this treatment well and good outcomes are achieved.