Background: Myelofibrosis is a chronic myeloproliferative neoplasm characterized by progressive bone marrow fibrosis and extramedullary hematopoiesis leading to splenomegaly. Progressive splenomegaly is common in advanced myelofibrosis, and spleen volume is a predictor of survival. The oral selective small-molecule JAK 1/2 inhibitor ruxolitinib is used for symptomatic management of intermediate-2 and high-risk myelofibrosis, and leads to significant reduction in spleen size. Ruxolitinib is usually discontinued prior to hematopoietic stem cell transplantation (HSCT). Recent studies suggest continuing ruxolitinib in the peri-transplant period may prevent tumor lysis and cytokine dysfunction due to ruxolitinib withdrawal, as well as improve bone marrow microenvironment for successful stem cell engraftment.CT-based determination of spleen volume was traditionally calculated based on maximum and craniocaudal length, width, and thickness, and correlated with sonographic measurements. However, 3D volumetric analysis of splenic volume is a quantitative imaging biomarker that can provide increased accuracy and detect subtle changes in surface contour. We describe 3D spleen volumetry in patients with myelofibrosis receiving non-myeloablative hematopoietic stem cell transplantation (HSCT) with concurrent ruxolitinib therapy.
Methods: This is a retrospective single center study of 29 patients (16 male, 13 female) with primary or secondary myelofibrosis treated with reduced-intensity allogeneic HSCT and peri-transplant ruxolitinib therapy at the City of Hope Helford Clinical Research Hospital between 2003-2017. Eligible patients were selected based on availability of abdominal CT images obtained prior to starting treatment and at least one month after transplantation. Suitable images were processed for 29 of 102 transplant patients screened. Voxel-based volumetric data was compiled with 3D imaging software (GE AW server 3.2) by contouring the spleen on serial axial images and evaluating program-generated histograms.
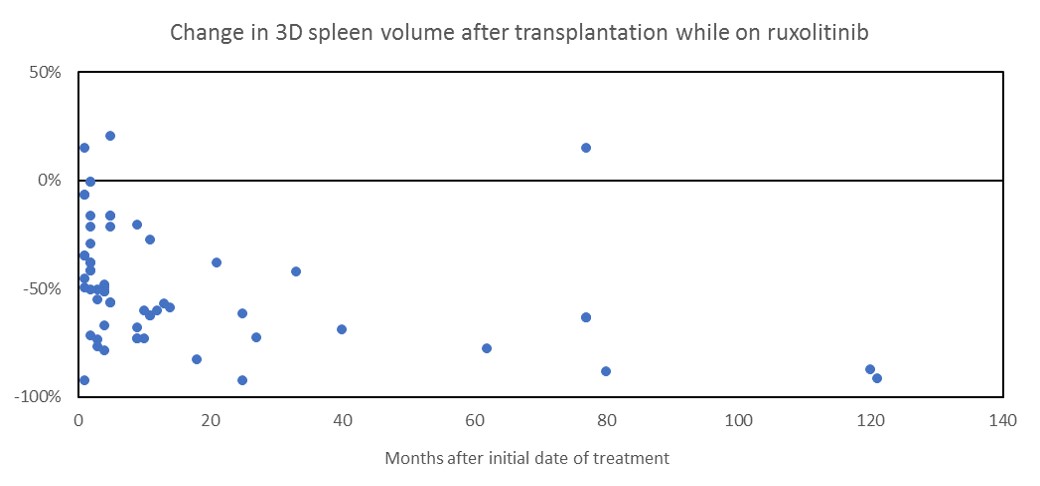
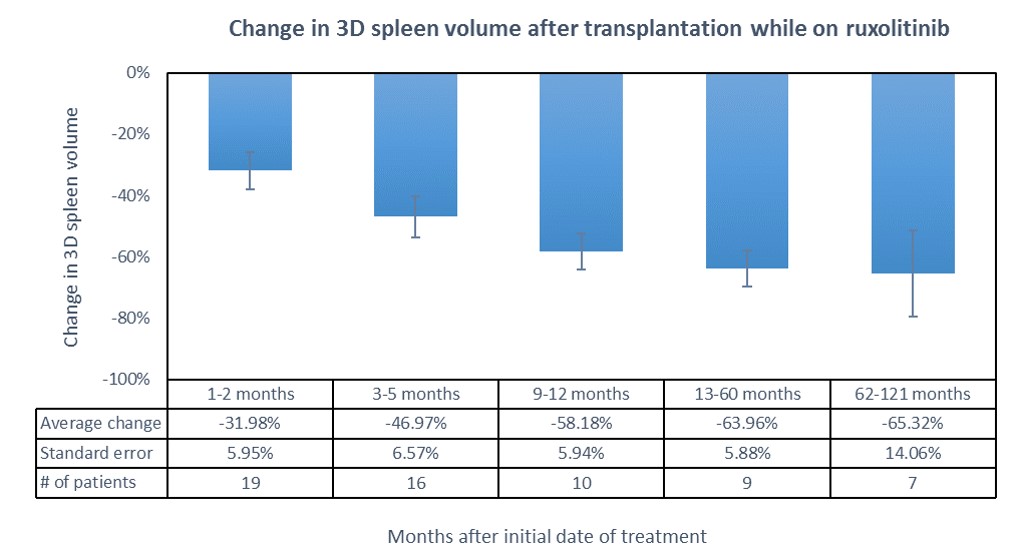
Results: Volumetric analysis of 3D spleen volume was assessed for 29 patients with a median age of 56.6 (41 to 70 years). The median time from date of treatment to the first follow-up CT was 4 months. Median change in spleen volume was -49.8% reduction (-92.4% to +14.8%). Only two patients (6.9%) developed an increase in spleen size. There was no significant difference between genders.
Conclusions: We used quantitative imaging software to measure 3D splenic volume in patients receiving ruxolitinib therapy and undergoing HSCT. There was significant reduction in spleen volume with HSCT + peri-transplant ruxolitinib. Advanced 3D volumetric analysis allows more accurate quantification of splenic volume than traditional 2D ultrasound-based calculations, and may provide useful information including perfusion and surface contour. Further studies are needed to identify additional quantitative imaging biomarkers can be used to detect disease progression, monitor response to therapy, and predict prognosis.