Background: Low serum concentrations of tetracycline antibiotics may raise concerns on their efficacy in patients with secondary bacteremia, especially in comorbid patients and in those with higher acuity of illness. Omadacycline (OMC), an aminomethylcycline antibiotic, showed non-inferiority to linezolid (LZD) in two acute bacterial skin and skin structure infections (ABSSSI) studies (Omadacycline in Acute Skin and skin structure Infections Study [OASIS]-1 and 2), or to moxifloxacin (MOX) in a community-acquired bacterial pneumonia (CABP) study (Omadacycline for Pneumonia Treatment In the Community study [OPTIC]). This analysis considers clinical outcomes in patients with secondary bacteremia identified in these studies.
Methods: Baseline blood cultures were taken from adult patients enrolled in Phase 3, randomized, double-blind clinical studies of OMC. Patients received ≥1 treatment dose: N=1347 ABSSSI (OMC n=676; LZD n=671); N=774 CABP (OMC n=386; MOX n=388). Efficacy was evaluated by investigator assessment of clinical response at a post treatment evaluation (PTE) 7-14 days (ABSSSI) or 5-10 days (CABP) after last dose.
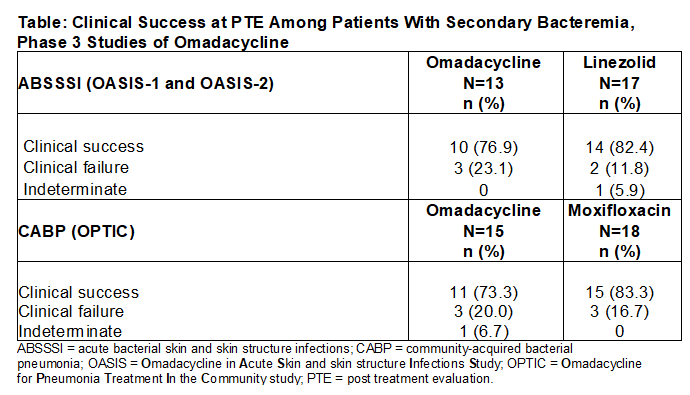
Results: Bacteremia was confirmed in 63 patients (3%) across the three studies. Staphylococcus aureus was the most common pathogen in patients with ABSSSI (N=30: OMC n/N=7/13; LZD n/N=9/17), with median treatment durations of 9 days (OMC) and 10 days (LZD). Streptococcus pneumoniae was the most common pathogen in patients with CABP (N=33; OMC n/N=11/15; MOX n/N=11/18), with median treatment durations of 11 days (OMC) and 14 days (MOX). Clinical success was numerically comparable between treatment groups within each study (Table) after completion of therapy.
Conclusions: The clinical successes of patients receiving OMC for ABSSSI or CABP with secondary bacteremia were comparable to results for LZD and MOX, respectively. Although limited by the small sample size, data from ABSSSI and CABP study patients with secondary bacteremia provide reassurance for use of OMC. Real-world OMC data in patients with ABSSSI and CABP will be needed to assess outcomes in patients with additional comorbidities and/or higher acuity of illness.