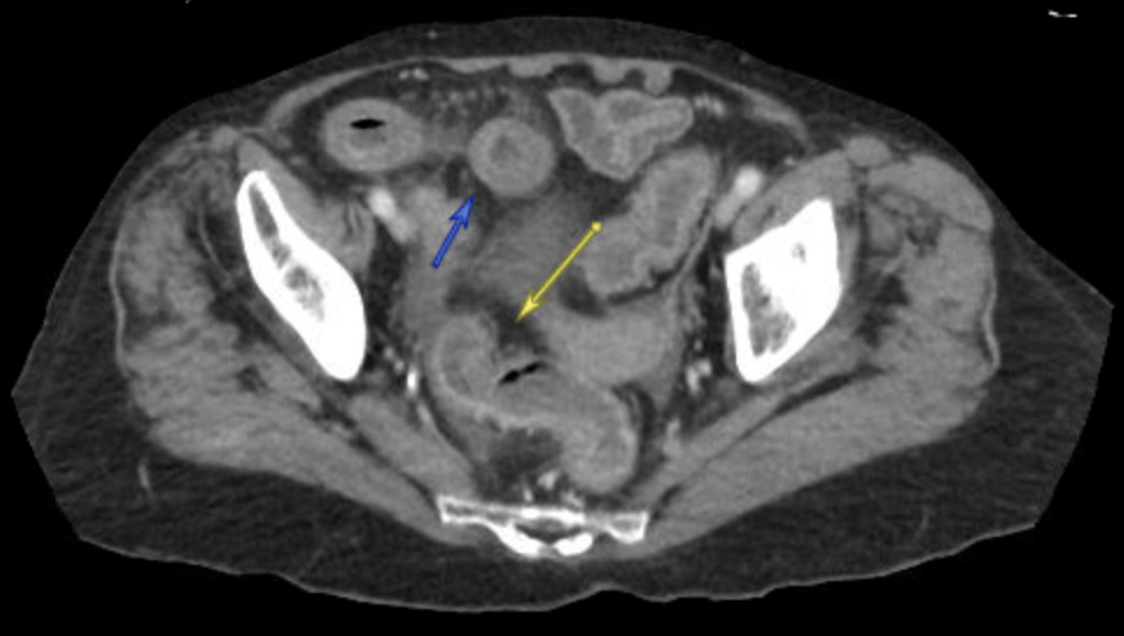
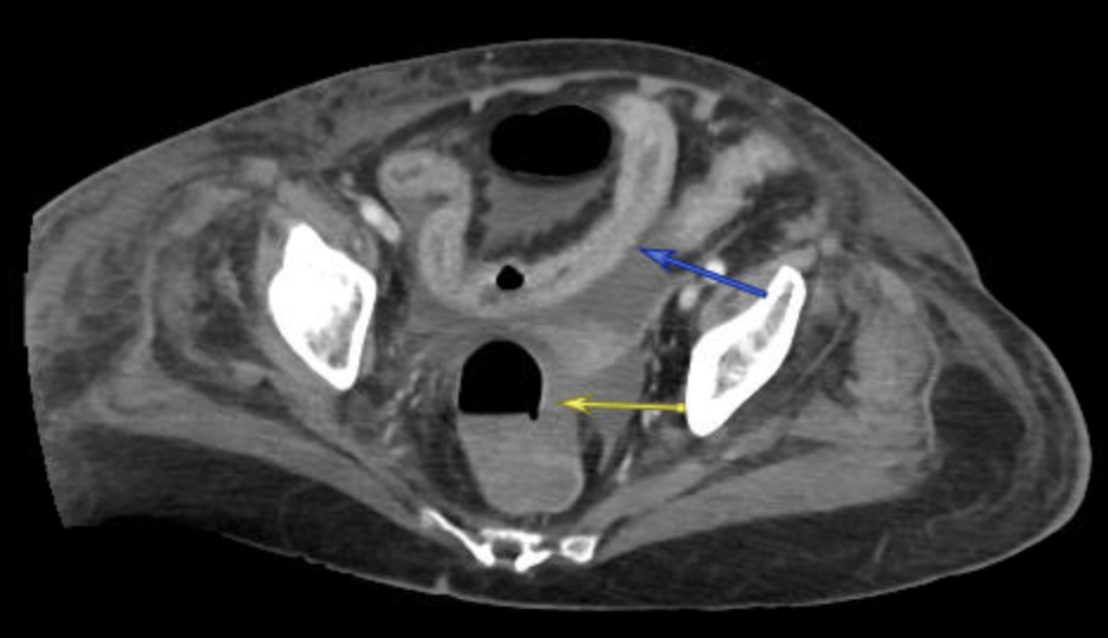
Case Presentation: A 77-year-old female with breast cancer on capecitabine (Xeloda) initiated 3 weeks ago presented with mouth pain and diarrhea for the last 10 days. She denied any fevers, sick contacts, or recent antibiotic use. Her last dose of capecitabine was 1 week prior. On arrival, she was hypotensive and tachycardic; physical exam was significant for diffuse abdominal tenderness and oral ulcers. Labs were notable for severe neutropenia (ANC 0.06 cells/µL). CT showed diffuse enterocolitis (figure 1) and she was started on broad spectrum antibiotics. Clostridium difficile (C. diff) testing was positive and she was transitioned to oral vancomycin and piperacillin-tazobactam for suspected neutropenic enterocolitis. CT on day 5 showed persistent enteritis with resolved colitis. Her neutropenia resolved with filgrastim but her diarrhea continued despite an improved abdominal exam. It was suspected her symptoms might have been capecitabine-induced when CT on day 15 showed persistent inflammatory changes in the distal ileum (figure 2). Dihydropyrimidine dehydrogenase (DPD) testing was positive for one gene variant, DPYD intermediate metabolizer. Colonoscopy with biopsy was negative though ileoscopy was unsuccessful due to stool burden. Oral vancomycin was replaced with fidaxomicin in addition to aggressive antimotility agents and supportive care. Her diarrhea slowly improved and she was discharged on day 30.
Discussion: Diarrhea is a known side effect of capecitabine, a drug listed on the WHO Model List of Essential Medicines. While DPD deficiency is rare (1-7%), individuals with DPYD gene mutations are at increased risk of life-threatening reactions from fluoropyrimidines, including severe diarrhea, mucositis, and neutropenia (1). This patient’s presentation could be explained by capecitabine toxicity and further exacerbated by C. diff, leading to terminal ileitis and colitis. She had delayed improvement in symptoms despite resolution of her neutropenia and treatment for C. diff. Additionally, she had painful oral ulcers and persistent inflammatory changes in the terminal ileum. There have been cases describing terminal ileitis induced by capecitabine, confirmed with colonoscopy showing ileal ulceration (2). Uridine triacetate (Vistogard) has been approved to reverse the effects of capecitabine if used within 4 days of the last dose, though it has been shown to be beneficial even outside of this window (3). It is a safe and effective life-saving antidote with mild side effects, most notably nausea and vomiting (4). In this case, uridine triacetate was not given, perhaps due to anchoring bias from positive C. diff testing.
Conclusions: It is important to consider DPD deficiency as a major factor contributing to this patient’s acute illness. This case argues for uridine triacetate use as soon as there is suspicion for capecitabine toxicity, regardless of gene testing, which can delay treatment. While the European Medicines Agency recommends testing for DPD deficiency prior to initiating treatment with fluoropyrimidines, such a recommendation does not exist in the United States. This case argues for a similar guideline to be adopted in the United States as standard of care to prevent life-threatening capecitabine toxicity.