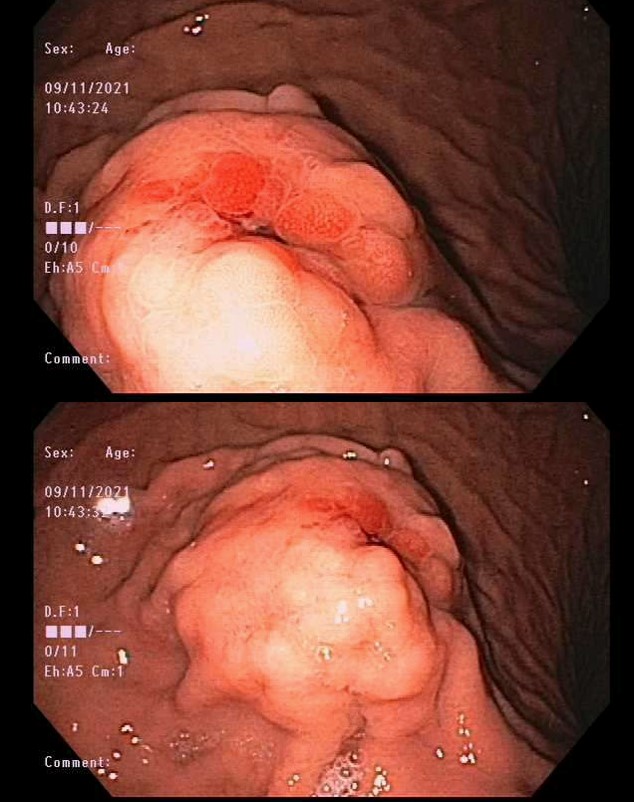
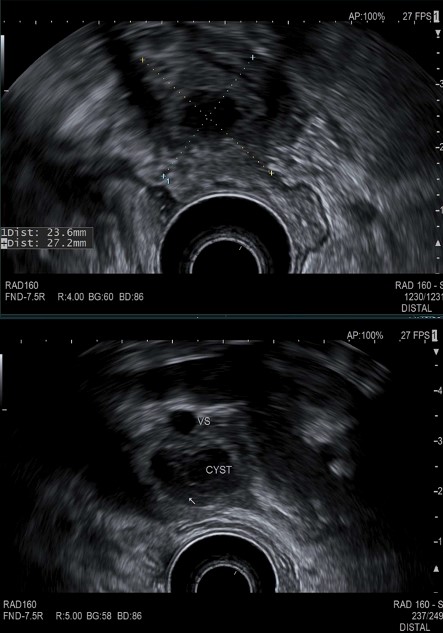
Case Presentation: A 35-year-old male with a history of SPINK-1-associated hereditary pancreatitis presented to the emergency department with hematemesis. Hemoglobin (Hgb) of 11.2 g/dL (Baseline Hgb 15g/dl), and a lipase of 51 U/L were noted on admission. The patient was started on intravenous (IV) pantoprazole and esophagogastroduodenoscopy (EGD) was performed revealing a 3cm polypoid lesion with ulceration and stigmata of recent bleeding on the greater curvature of the gastric body (Figure 1). Histology of the ulcer bed was consistent with ulcer formation in the background of epithelial reactive changes. No evidence of Helicobacter pylori, metaplasia, dysplasia, or malignancy was reported. Hospital course was complicated by worsening anemia to a Hgb level of 8.2g/dL.Repeat EGD and endoscopic ultrasound (EUS) was performed and revealed a 25mm by 21mm cystic mass with evidence of muscularis propria involvement (Figure 2). There was no echogenic border between the cystic process and the splenic vein. Calcifications, hyperechoic strands, lobularity, and shadowing was noted throughout. Fine needle biopsy was performed, and pathology was unrevealing. CT angiography also showed the pancreatic tail cystic mass appearing to invade the greater curvature of the stomach while directly abutting the splenic vein. There was no evidence of acute pancreatitis, contrast extravasation or sizeable arterial branching nearby.
Discussion: Chronic pancreatitis is rarely caused by hereditary pancreatitis, which is typically caused by mutations in genes regulating serine protease expression such as SPINK-13. Patients with these mutations are at increased risk of complications ranging from pancreatic pseudocyst formation to pancreatic adenocarcinoma (4). Pseudocyst formation is a well-described phenomenon, occurring in 20-40% of patients with chronic pancreatitis (5). Pseudocyst hemorrhage in the setting of chronic pancreatitis has an incidence of 6-17% (6). Even rarer is gastric bleeding from such a pseudocyst.In a case series by Sødenaa and Sørenide, 27 cases of gastric hemorrhage secondary to pancreatic pseudocyst were reviewed, with 19 of the 27 being of splenic artery origin. The others had bleeding from the left gastric, gastroduodenal, inferior pancreaticoduodenal, or transverse pancreatic arteries. While there is ample literature discussing pancreatic pseudocyst hemorrhage due to involvement with neighboring arteries, we have found no literature regarding splenic vein involvement. Venous hemorrhage typically presents more indolently, causing insidious blood loss. Because of this, emergent surgery or intravascular embolization was considered but not deemed urgent, in contrast to life-threatening massive arterial bleeding. Overall, indications for splenic vein embolization (as opposed to arterial embolization) are sparse. Splenic vein embolization for hemorrhage was not recommended, as this approach would not be expected to control hemorrhage in our case. Additionally, increasing pressure behind the embolization zone could presumably result in worsening of bleeding long term by creating a pathologic process similar to sinistral portal hypertension.
Conclusions: To conclude, pancreatic pseudocyst erosion of the splenic vein and gastric wall causing gastrointestinal bleed is a very rare phenomenon, even more so as a sequela of hereditary pancreatitis. As such, treatment is limited, but surgical management through distal pancreatectomy, splenectomy, and partial gastrectomy is indicated.