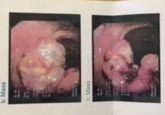
Case Presentation: 78 year old Asian female with a history of diabetes mellitus type II, hypertension, and recently diagnosed colon cancer presented with severe left ear pain and vesicular rash associated with left sided hearing loss and left facial paralysis. She endorsed decreased taste sensation as well as poor appetite resulting in twenty pounds of weight loss over the past few months. She was able to close both her eyes and denied vertigo and tinnitis. On physical exam, the erythematous left pinna had dark vesicular lesions with yellow exudate with edema of the antihelix. Tympanic membrane was intact with some clear secretions within the left ear canal. Lab was significant for mild anemia with hemoglobin of 11.2. She was diagnosed with herpes zoster oticus (Ramsay Hunt syndrome). She was treated with intravenous acyclovir and prednisone 60 mg once a day for 4 days with subsequent tapering with marked improvement in ear swelling. She was referred to oncology and colorectal surgery for treatment of ascending colon cancer seen on colonoscopy ten days prior to admission.
Discussion: Herpes zoster oticus is caused by reactivation of the varicella zoster virus. Varicella zoster virus has been detected in the trigeminal and geniculate ganglion.1 Our patient had the classical symptoms of unilateral ear pain with vesicular rash and facial paralysis. She had immunosuppression due to colon cancer which increased her risk for herpes zoster oticus.
Conclusions: She was treated with both acyclovir and prednisone. However, there is no large randomized controlled trial to guide therapy. One retrospective study of 101 patients in a case series reported that dual therapy with antiviral and steroids begun at or after day 5 of the illness had best facial nerve outcomes in 1 year as measured by improvement in House-Brackmann grade units. 2 A Cochrane Database systematic review described a small study with 15 patients which found no difference between IV acyclovir and steroids compared to steroids alone. 3 On October 25, 2017 the Advisory Committee on Immunization Practices voted that Shingrix, zoster vaccine recombinant adjuvanted, be recommended for healthy adults aged 50 years and older to prevent herpes zoster (shingles).4 Compared with placebo, this vaccine reduced the risk of developing herpes zoster by 97.2% in people 50 years and older. 5 The shingles vaccine should not be given to people with weakened immune system due to AIDS, cancer such as leukemia or lymphoma, and women who are or might be pregnant.4
1 Furuta Y, Takasu T, Fukuda S, Sato-Matsumura KC, Inuyama Y, Hondo R, Nagashima K. Journal Infectious Disease 1992;166(5):1157
2 Coulson S, Croxon GR, Adam R, Oey V. Otology and Neurotology 2011 August: 32(6): 1025-1030
3 Uscategui T, Doree C, Chamberlain IJ, Burton MJ. Cochrane Database Syst Rev 2008
4 https://www.cdc.gov/vaccines/vpd/shingles/public/index.html
5 Prescribing information of Zoster Vaccine Recombinant, Adjuvanted.