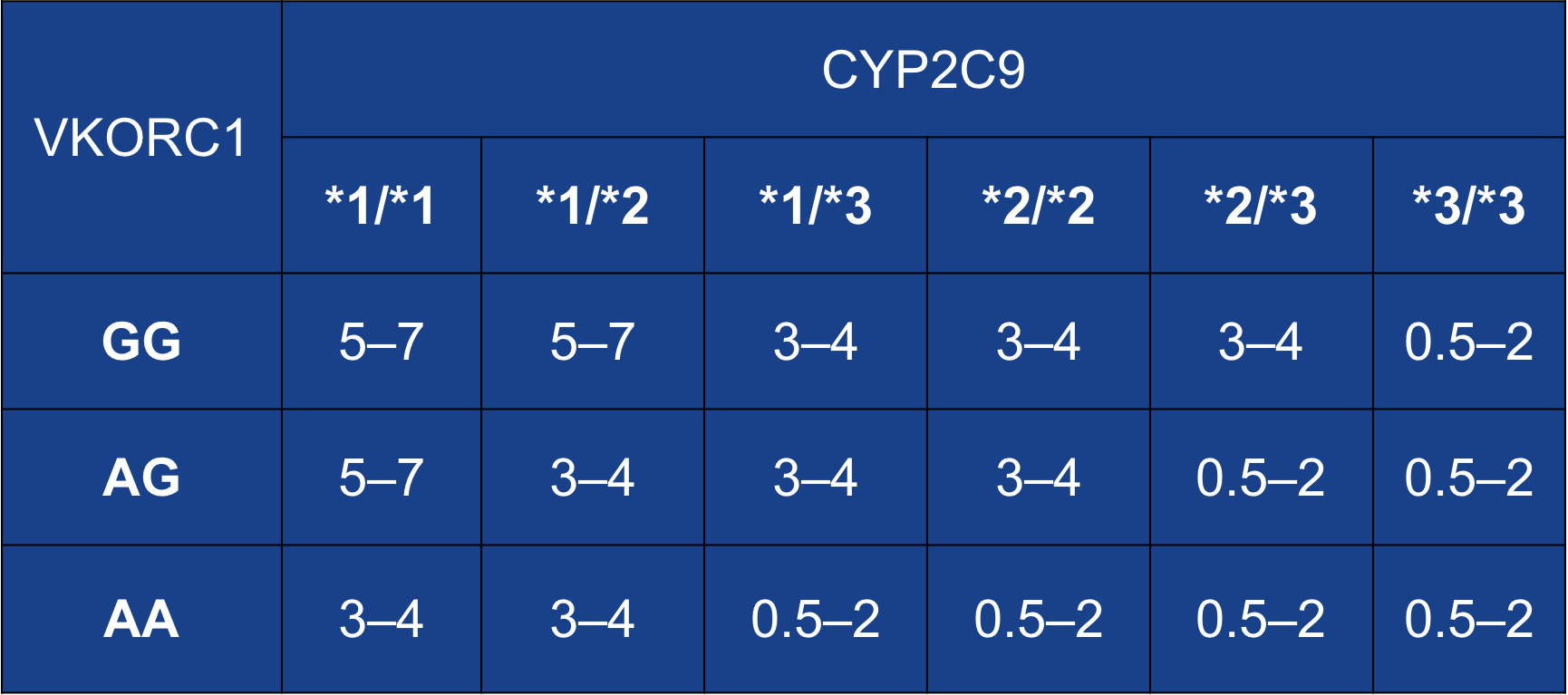
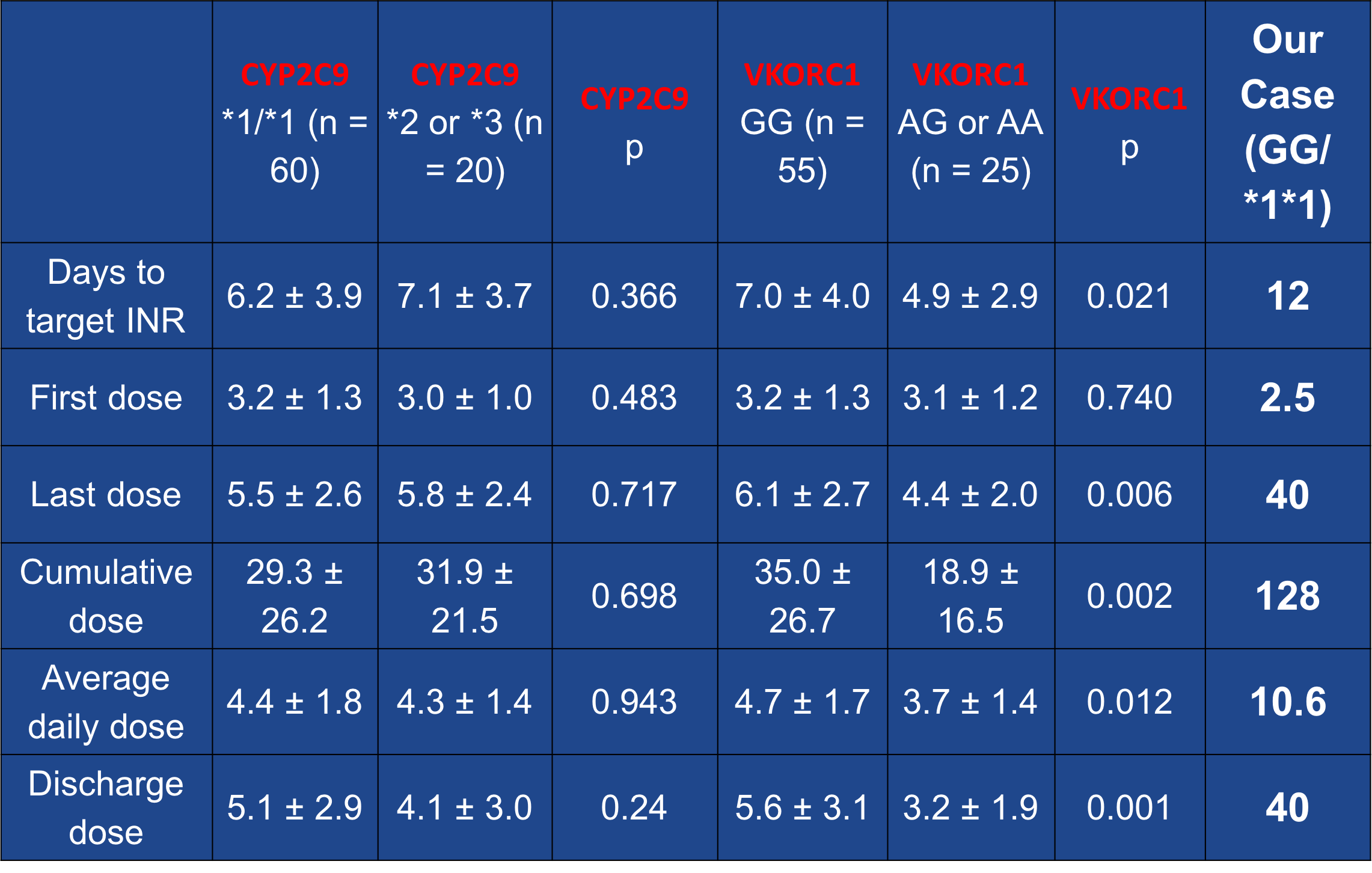
Case Presentation: 48-year-old African American (AA) male with history of Non-ischemic cardiomyopathy on milrinone (EF: 15%), after CHOP therapy for his lymphoma, diabetes mellitus, polysubstance abuse was admitted with an acute exacerbation of chronic systolic heart failure with cardiogenic shock. He was being managed with milrinone drip and lasix in the CCU and considered a candidate for LVAD implantation. He underwent durable HVAD LVAD implantation and TVR, antiplatelet was initiated with aspirin 325 mg daily, anticoagulation therapy with warfarin was started at 2.5 mg/day on post-op day 4 once the surgical drains were out with an INR goal of 2-3. The INR remained sub-therapeutic at 1.1 and LDH started trending up from 412 to 469 U/L so continuous heparin drip was started. After confirming patient compliance and making sure he was not taking any medications or foods (Vitamin K rich diet)that could increase warfarin metabolism, its dose was increased. Warfarin was increased in small increments on an almost daily basis from 2.5 to 4 to 5 to 7.5mg to 10 to 20 and INR remained at 1.2. Gene testing for VKORC1 -1639 (A/G) and CYP2C9 (*1/*2/*3) allelic variants were ordered. He tested positive for this resistance, positive for VKORC1 (G/G) and CYP2C9 (1/1) alleles and was negative for CYP4F2*3 genotype 1/1 (rs2108622T) allele. This prompted us to increase his dose from 20 to 30 and then 40 mg to achieve therapeutic INR. He finally became therapeutic (INR: 2.1) at 40 mg/daily of Coumadin. His VAD was noted to do well and he was discharged on this dose of warfarin with close follow-up in clinic.
Discussion: Continuous-flow left ventricular assist devices (CF-LVAD) require the use of warfarin-based anticoagulation, which is proven to be effective in prevention of thromboembolic complications. However, warfarin has a narrow therapeutic index and a wide variation in individual dose requirements. Growing lines of evidence suggest that polymorphisms in two genetic variants involved in warfarin pharmacodynamics and pharmacokinetics (vitamin K epoxide reductase complex submit 1 [VKORC1] and P450-2C9 (CYP2C9) respectively) account for the associated resistance. Functional warfarin “resistance” has been defined as warfarin requirements of more than 15 mg/day to achieve therapeutic anticoagulation or failure to achieve therapeutic anticoagulation with more than 20 mg/day.
Conclusions: – Given the high prevalence of bleeding and thrombotic events in CF-LVAD patients, routine genotyping before device implantation can potentially lead to safer and more effective anticoagulation. – It will allow for avoidance of excessive doses in warfarin sensitive patients, and shortening of bridging time in warfarin resistance patients.