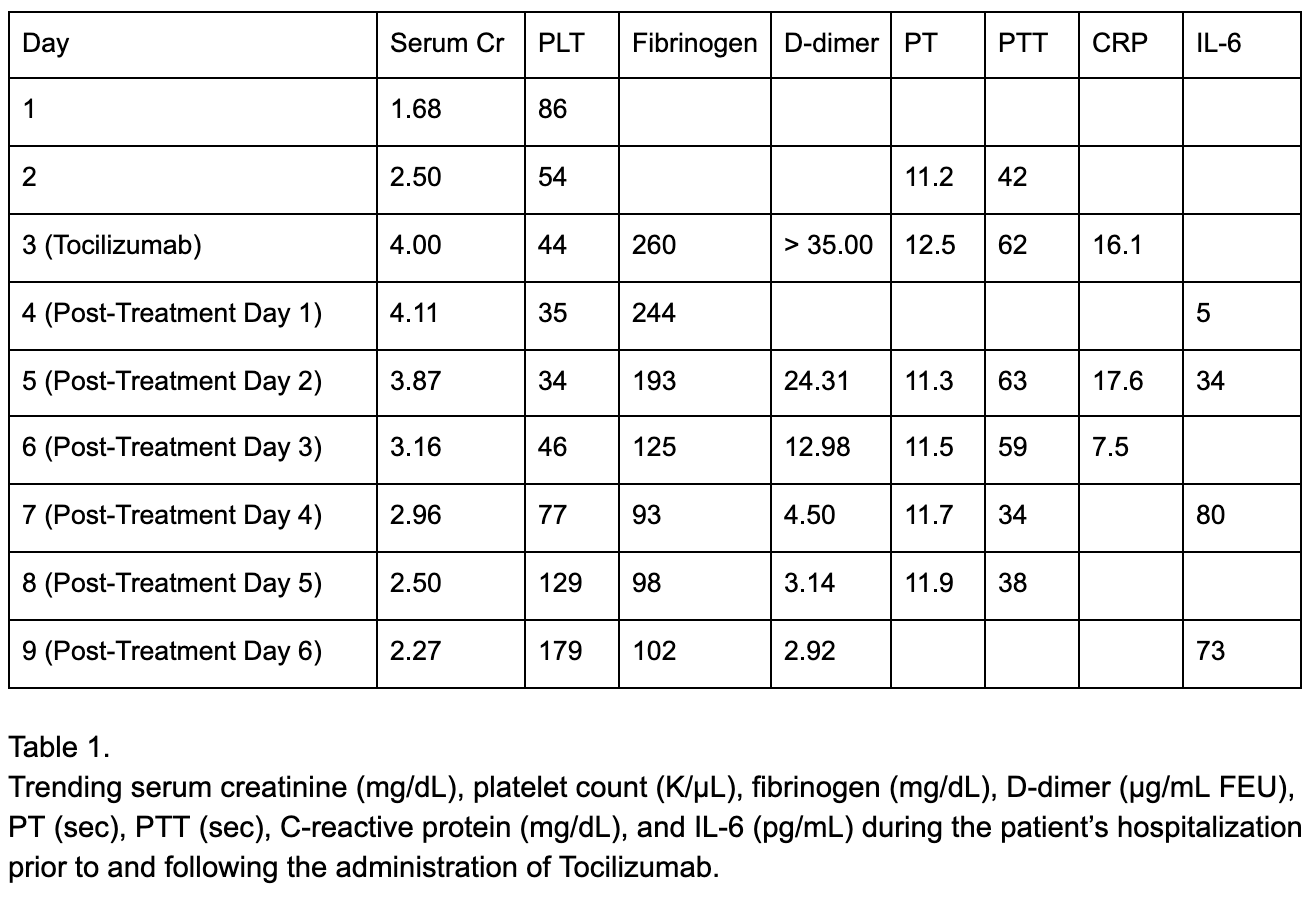
Case Presentation: We present a 71-year-old male with stage IV melanoma on cycle 17 of Nivolumab admitted after presenting with altered mental status, hypotension, tachycardia, fever of 40.5°C, hypoxia requiring BiPAP, and grade 3 maculopapular rash. Labs revealed serum creatinine of 1.68, platelet count of 86, fibrinogen of 260, PT 11.2, PTT 42, d-dimer > 35, and CRP of 16.1 (table 1). Vancomycin and piperacillin-tazobactam were started for suspected sepsis, followed by methylprednisolone after 24 hours with no improvement due to growing concern that his symptoms were immune-mediated. After another 24 hours without improvement, tocilizumab was administered for suspicion of cytokine release syndrome (CRS). Within one hour, fever and hypotension resolved, and hypoxia and mental status improved. In the following days, platelet count and kidney function significantly improved as well. Decreased CRP suggested blunting of inflammatory response and increased IL-6 from IL-6 receptor blockade were also seen. He was discharged from the hospital on post-treatment day 6 and followed up one week later at his baseline level of function.
Discussion: Immune checkpoint inhibitors such as CTLA-4 and PD-1 inhibitors mediate T cell induced tumor cell destruction by blocking malignant cells’ ability to negatively regulate T cells [1]. Significant responses have been seen across a broad spectrum of malignancies ushering in a new era of cancer treatment. In addition to its anti-tumor effect, checkpoint inhibition can lead to loss of maintenance of self-tolerance, leading to immune-mediated adverse events (irAEs) [2]. These events can affect any organ, with pneumonitis, hepatitis, and colitis among the most commonly reported events. A rare, life-threatening reported side effect is CRS. CRS is a systemic inflammatory response described in a number of immunotherapies including CAR T cell and bispecific T cell engagers (BiTEs). The primary pathophysiology involves activation of immune and nonimmune cells, leading to massive release of inflammatory cytokines [3]. CRS can present as a flu-like syndrome or as an overwhelming systemic disease characterized by hypotension, DIC, and multi-organ failure [4]. IL-6 appears to play a major role in CRS through activation of the complement and coagulation cascades [5]. The IL-6 receptor inhibitor tocilizumab is the only FDA-approved treatment for CRS. There have been few reported cases of checkpoint inhibitor-induced CRS in the literature, including in a 29-year-old with sarcoma [6], and a 25-year-old with Hodgkin’s Lymphoma [7]. CRS developed early on in treatment in these cases, and our case now shows the potential for CRS in nivolumab therapy occurring months after initiation of treatment.
Conclusions: CRS is a rare, life-threatening condition that requires early diagnosis and management. Though initial investigations may point to a more common condition such as sepsis, a low threshold for consideration of irAEs and CRS should exist where the clinical picture warrants. It is likely that many cases exist in which the diagnosis is missed, and appropriate treatment not given due to lack of clinical awareness. Physician education, prompt implementation of specific testing, and treatment with tocilizumab can lead to significantly improved outcomes as our case shows. The patient passed away after a second CRS event refractory to tocilizumab two months after our admission, which reinforces the need for continued research for additional treatment options for CRS, especially in recurrent cases.