Background: Pulmonary arterial hypertension (PAH) is a progressively debilitating disorder characterized by sustained increases in pulmonary vascular resistance and pulmonary arterial pressure, which eventually leads to right-sided heart failure. Current therapies for PAH work predominately as vasodilators to target symptoms, rather than focusing on the initial signals that promote disease progression. These therapies have proved ineffective at improving patient outcomes, with a 50 – 60% three-year survival rate post-diagnosis. Therefore, identifying early changes in the pathophysiology of PAH is essential for designing more effective therapeutics. Endothelial–to–mesenchymal transition (EndMT), a process implicated in PAH progression and severity, contributes to the loss of endothelial markers and a concomitant increase in mesenchymal markers. EndMT leads to endothelial dysfunction, involving aberrant cell proliferation, enhanced migration and invasion, and a pro-inflammatory phenotype. However, the mechanisms driving this process have yet to be fully elucidated. Recently, intracellular osteopontin (iOPN) has been shown to reverse the effects of epithelial–to–mesenchymal transition (EMT). EMT is well-studied, particularly in cancer, and evidence is mounting that EndMT parallels EMT in multiple ways. Additionally, secretory osteopontin (sOPN) is a well-known pro-inflammatory, pro-metastatic cytokine. Therefore, the goal of this study was to investigate the phenotypic characteristics and protein levels of both iOPN and sOPN in wild-type pulmonary arterial endothelial cells (WT-PAECs) and PAECs derived from a Sugen hypoxia rat (SURAT) model of PAH.
Methods: Pulmonary artery endothelial cells came from both control Fischer rats and rats exposed to the Sugen hypoxia model of PAH. Functional assays were conducted using cells grown to full confluence. Both iOPN and sOPN levels were assessed using western blot analysis and band densitometries were quantified using ImageJ. Wound healing and modified Boyden chamber assays were conducted to assess migration and invasion. An endothelial network formation assay was performed to assess angiogenic capacity, and a foci formation assay was conducted to assess proliferation.
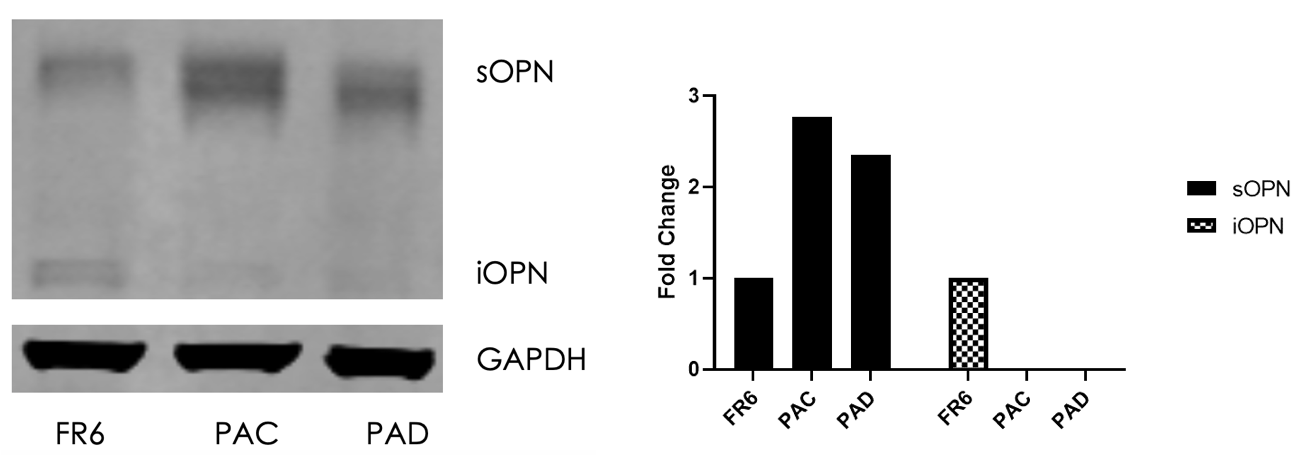
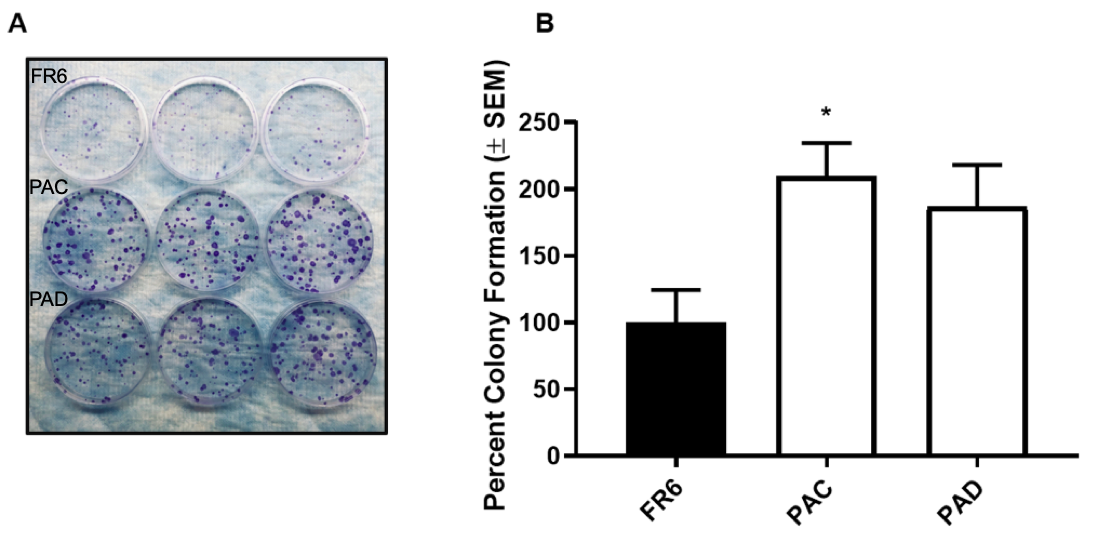
Results: Western blot analysis revealed that, compared to the WT, SURAT PAECs demonstrated a significant increase in sOPN and concomitant decrease in iOPN protein. An endothelial network formation assay demonstrated that SURAT PAECs were more angiogenic than WT PAECs. Wound healing assays showed that SURAT PAECs possessed a higher migratory capacity compared to WT. Invasion assays demonstrated that SURAT PAECs were more invasive compared to WT. Finally, foci formation assays revealed that SURAT PAECs exhibited a hyperproliferative, anti-apoptotic phenotype compared to WT.
Conclusions: Taken together, we conclude that a loss of intracellular osteopontin and concomitant increase in sOPN is a potential driver of the EndMT phenotype seen in PAH.