Background: Methicillin-resistant Staphylococcus aureus (MRSA) colonization of hospitalized patients is associated with higher readmission rates and increased morbidity. Depending on the mechanisms of transmission, numerous potential control interventions exist to reduce the burden of disease. These interventions include decolonization, improving hand-washing adherence, and enhanced environment cleaning. To evaluate the impact of various types of control, it is imperative to first quantify the underlying transmission patterns, and the degree of superspreading (i.e., the tendency of some cases to transmit much more than others). Given the preponderance of asymptomatic colonization, it can be challenging to quantify the relative importance of different transmission mechanisms and assess control efficacy. Fortunately, by identifying clusters of transmission, whole genome sequencing (WGS) provides an opportunity to overcome these challenges.
Methods: We applied cluster analysis techniques to published MRSA WGS data for a 12-month period in the East of England [Coll et al.]. Subsequent application of a maximum likelihood branching process model permits and assessment of MRSA prevalence, transmissibility, the degree of transmission heterogeneity and the potential effectiveness of control. Our model builds upon previous work that showed a direct relationship between the size distribution of infection clusters, the effective reproduction number (R) and the dispersion parameter (k) [Blumberg et al.]. Targeted control was modeled by reducing R for a proportion of cases, where cases prone to superspreading were prioritized for interventions. Universal control was modeled by reducing R for all cases.
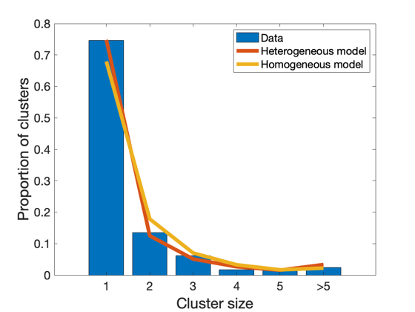
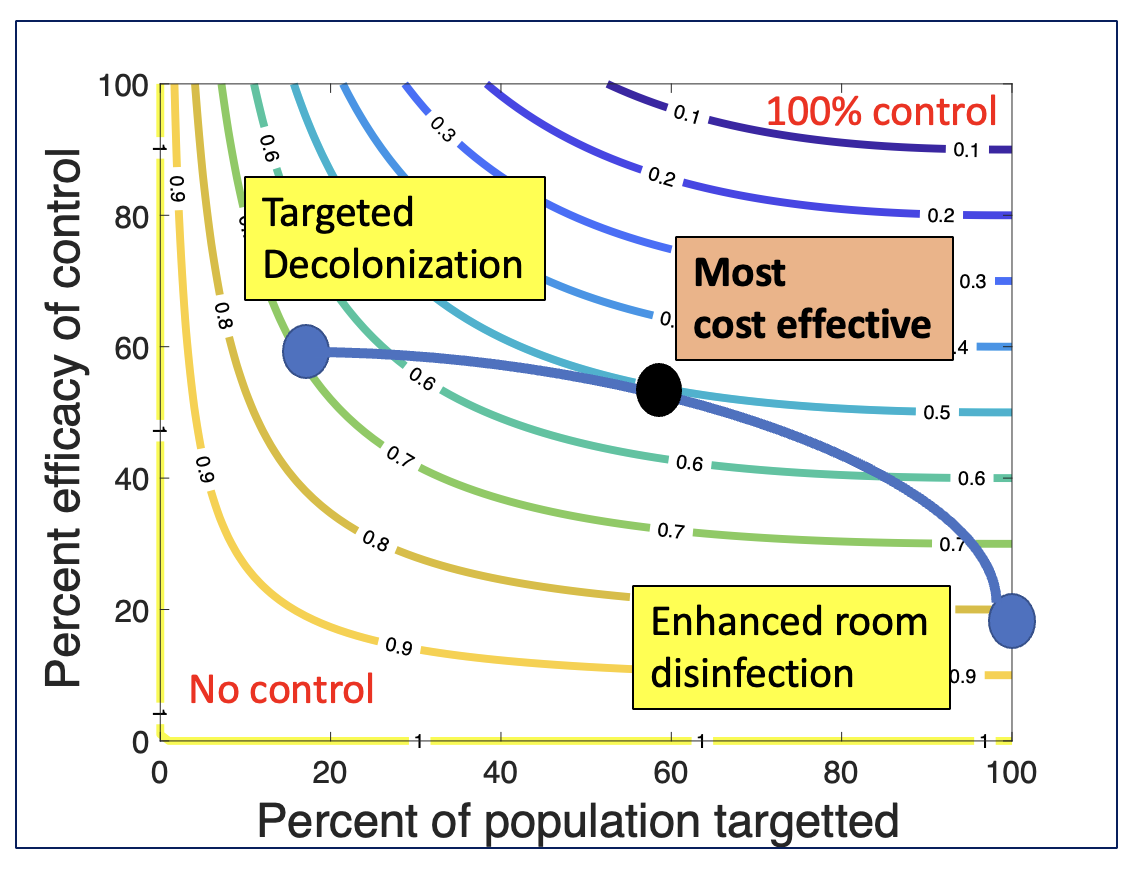
Results: The effective reproduction number for the East of England data is estimated as 0.38 (95% CI: 0.35-0.43, Figure 1). The dispersion parameter is estimated as 0.53 (0.36-0.86) reflecting a high amount of superspreading. We infer that universal control with 20% effectiveness (e.g., enhanced environmental cleaning) could have decreased overall burden of MRSA by 8% (95% CI: 7 – 9%) during the time period of the study. Targeted control of 20% of cases with 60% efficacy (e.g., decolonization of high-risk individuals) could be as effective as universal control for all isolates (Figure 2). However, the most cost-effective solution is likely a combination of targeted and universal control.
Conclusions: WGS is a valuable tool for assessing the strength and superspreading potential of healthcare-associated disease transmission. It also permits comparison of infectious disease dynamics over time and for different locations. Meanwhile, the high degree of superspreading seen in healthcare-associated MRSA transmission helps to elucidate underlying mechanisms of transmission and accentuates the cost-effectiveness of targeted control. It also motivates the need for more detailed mechanistic modeling of hospital-based MRSA transmission that integrates patient specific factors, movement data and genome sequencing. Such models could be used to forecast which patients are at greatest risk for either acquiring or transmitting MRSA, thereby improving targeted control. Development of such technologies expands the opportunities to reduce the burden of antimicrobial resistant infections and improves capacity for responding to future infectious disease threats.