Background: In an effort to combat the national opioid crisis, our state legislation passed House Bill 451 effective July 1, 2019. It addresses medication overprescribing to decrease morbidity and mortality from opioid misuse, abuse, and overdose. The law has several requirements including discussion of non-opioid treatment alternatives, review of the Prescription Monitoring Drug Program (PDMP) database and providing an Alternatives to Opioid educational pamphlet prior to prescribing, ordering, dispensing or administrating a schedule 2 controlled substance. When discharging inpatients, a 3 day supply of opioids recommended with up to 7 days permissible with documentation of acute pain exception. Here, we describe the development and implementation of processes to meet the state House Bill 451 requirements and examine changes in discharge prescriptions within an urban community-based hospital.
Methods: Institutional Oversight: A multidisciplinary AMPS (Assessing and Managing Pain Safely) committee comprising of medical, informatics, quality, and educational leadership was established to address opioid stewardship and data review.Education to Prescribers: Information was disseminated via email to medical staff and residents from the Chief Medical Officer (CMO) over three consecutive months (July through October). Education was grated each month, from overview to detailed requirements concerning safe and responsible opioid prescribing and adherence to the law. In-person education was also provided during a medical staff meeting.Leveraging Informatics: In November, feedback showed that accessing, reviewing and documenting the PDMP prior to each prescription was cumbersome. There was also variation in attestation of patient education and discussion and provision of the pamphlet. Consequently, documentation tools were designed and direct access to the PDMP and educational materials were embedded into the electronic health record. Point of care decision support for opioid prescribing was leveraged in the the hospital electronic opioid ordering system. Prescription Monitoring: Quarterly reports of all inpatient discharge prescriptions were generated by Pharmacy Informatics. Outliers, defined as opioid prescriptions for >7 days or > 42 tablets (or equivalent), were extracted and reviewed by the committee.
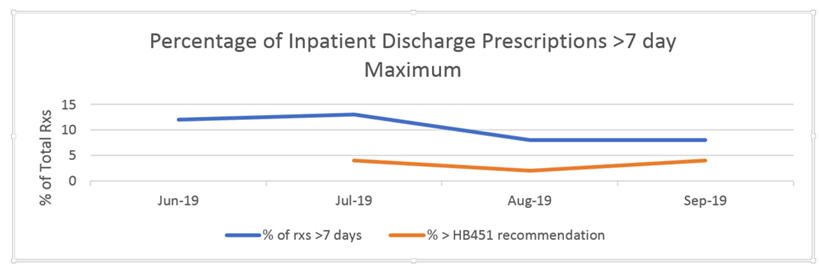
Results: In the 3 months prior to July, an average of 14% of opioid prescriptions were considered outliers. This decrease to 13% in July and for the next two months, was 8% (z=3.04,p<0.05). In most cases, malignancy as etiology for pain treatment was not documented or chronic pain instead of acute pain was listed as reason for prescribing; 2% and 4% of prescriptions were considered true outliers. Individualized feedback was provided to those prescribers from the CMO. (See Figure). Compared to data from the previous year, there was a 40% decrease in the total number of opioid tablets prescribed.
Conclusions: Changing prescribing patterns due to required counseling of non-opioid alternatives treatments of acute pain meaningfully impacted prescribing behavior practices. Patient’s shared decision making with informed understanding of non-opioid alternatives and potential adverse effects of opioid use significantly decreased the quantity of prescribed opioids for all hospitalized patients at discharge.