Background: Poorly controlled pain in hospitalized patients is common and may lead to excessive opioid administration and adverse events. Ketamine is a reversible, non-competitive NMDA receptor antagonist which blocks glutamate excitatory transmission and provides analgesia with minimal respiratory effects. Dosing for procedural sedation and induction of general anesthesia ranges from 1-4.5 mg/kg. Consensus guidelines for the use of ketamine at infusion rates of 0.5-2 mg/kg/hour recommend prescribers receive formal training in the administration of moderate sedation and that patients receive intensive care monitoring.2 However, continuous ketamine infusion at lower doses (0.1-0.4 mg/kg/hour) may have lower risk of adverse events. A Low Dose Ketamine for Analgesia (LDKA) Policy (off-label use) was implemented in March 2018 (prior to the consensus statement) allowing for patients to receive LDKA if the patient continued to have poorly controlled pain despite optimal multimodal treatment strategies. A LDKA infusion was initiated per protocol at 0.1 mg/kg per hour and increased by 0.1 mg/kg per hour if adequate analgesia was not achieved, to a maximum of 0.4 mg/kg per hour. No formal sedation training or routine monitoring was required, unless otherwise indicated for patient condition. We hypothesized that LDKA could be administered in the general hospital ward setting without specialized training or routine monitoring.
Methods: Institutional Review Board approval was obtained and the requirement for informed consent was waived. Data was extracted from electronic medical records for patients receiving LDKA infusion per protocol between March 1, 2018 and June 1, 2019. Exclusions included critical care areas, palliative or end-of-life care, intravenous push without continuous infusion, or fixed rate continuous infusion. We herein report the patient/infusion characteristics and adverse events associated with LDKA infusion per protocol.
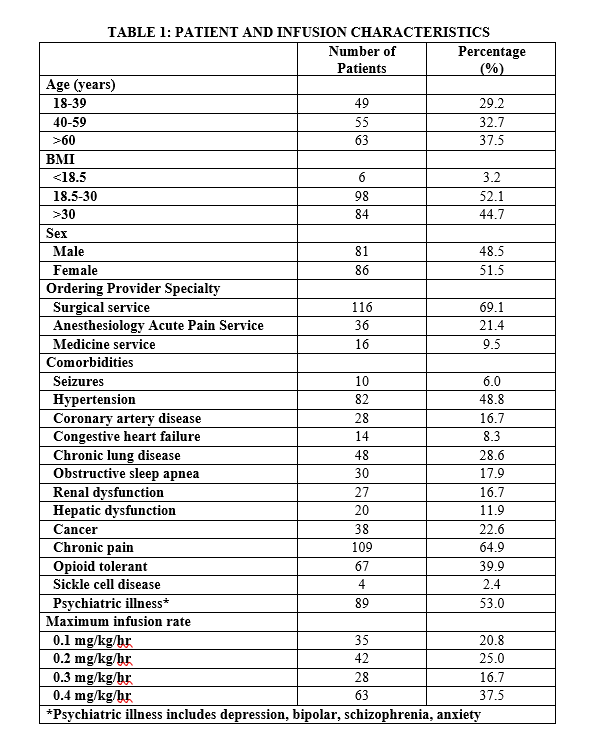
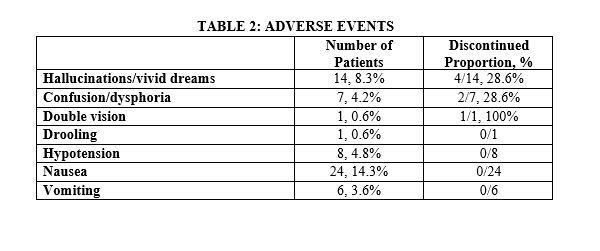
Results: LDKA infusion was administered in 168 patients in the general hospital ward setting and 80% were ordered by surgeons or hospitalists without formal sedation training (Table 1). The most common comorbidity in patients receiving LDKA was chronic pain (n=109, 65%) and psychiatric illness (n=89, 53%). Most patients (n=105, 62.5%) achieved adequate analgesia at infusion rates of 0.3 mg/kg/hour or less. Adverse events occurred in 47 patients (28%), but only 7 patients (15%) required discontinuation of LDKA infusion (Table 2). The most common adverse event was nausea (n=24, 14.3%), but the reasons for discontinuation were central nervous system effects (hallucinations, vivid dreams, confusion, dysphoria, and double vision). Although hypotension (defined as a 20% reduction in baseline with systolic blood pressure < 90) occurred in 8 patients, none were symptomatic or required discontinuation of the LDKA infusion. No respiratory depression episodes or other serious adverse events occurred.
Conclusions: LDKA infusion (at doses ranging from 0.1-0.4 mg/kg/hour) provides adequate analgesia when added to a multimodal treatment regimen and has low risk of serious adverse events in hospitalized patients on the general hospital ward without routine monitoring. Although adverse events occurred, there were not clinically significant episodes of hemodynamic instability and no respiratory depression events.