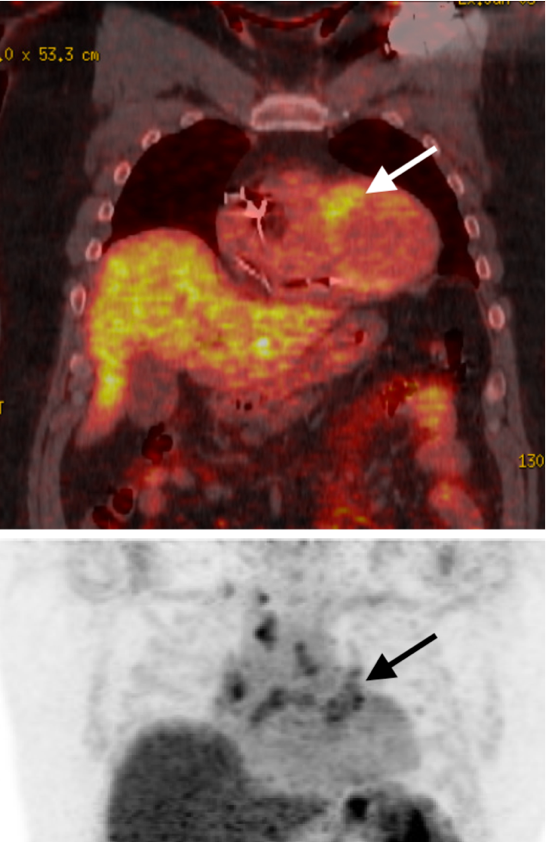
Case Presentation: 56-year-old male with a history of squamous cell cancer of forehead s/p Mohs surgery who was admitted from the outpatient cardiology stress lab after his EKG revealed complete heart block. Baseline TTE done during the initial part of the stress test revealed LVEF25-30%. Patient was asymptomatic on admission, hemodynamically stable with no laboratory abnormality. CXR unrevealing. Cardiology and EP were consulted. Given unclear etiology of new systolic HF, the patient underwent heart cath to rule out ischemic etiology. LHC revealed largely non-obstructive disease. LFTs/ESR/CRP/ferritin/TSH/Lyme titers were all unrevealing. A cardiac MRI completed revealed findings consistent with cardiac sarcoidosis in addition to extensive fibrosis and LVEF 51% (decreased LVEF on initial TTE suspected to be underestimated due to bradycardia, desynchrony). Given extensive myocardial scarring burden, EP recommended CRT-D placement which was ultimately placed. Pulmonology consulted. High-resolution CT (HRCT) Chest was completed which revealed no conclusive evidence of nodal or pulmonary sarcoidosis.Rheumatology consulted and in discussion with cardiology, decided to hold off on initiation of steroids or immunosuppression until outpatient FDG-PET scan was completed to determine if disease was active and if ongoing inflammation or fibrotic changes were present. Outpatient PET scan was consistent with active cardiac disease (figure 1). In collaboration with rheumatology and pulmonology, the patient was started on Methotrexate 15 mg weekly sub Q and Prednisone 40 mg daily with long term taper and prophylactic Bactrim for PJP.
Discussion: Cardiac sarcoidosis is detected in 2-7% of patients with sarcoidosis, we present a case of isolated cardiac sarcoidosis (ICS) without any extra-cardiac manifestation (2,6). This case highlights the importance of including ICS in the differential diagnosis of a young patient with conduction system disease and non-ischemic cardiomyopathy. Our case also highlights the need for better diagnostic criteria for cardiac sarcoidosis. In our patient, presumptive diagnosis of CS was made after cardiac MRI which was further confirmed by PET Scan. CS is a challenging diagnosis to make. There are two pathways to a diagnosis of Cardiac Sarcoidosis based on the Heart Rhythm Society criteria (HRS) (3). The first is histological diagnosis from myocardial tissue, however, this is complicated by the low yield of endo-myocardial biopsy because of patchy involvement in cardiac sarcoidosis (4). Another pathway is clinical diagnosis from invasive and non-invasive studies which involves three criteria. Pertaining to our patient, we were able to meet 2/3 criteria which included patchy uptake on dedicated cardiac PET (in a pattern consistent with CS) 2) Other causes for the cardiac manifestation(s) have been reasonably excluded (LFTs/ESR/CRP/ferritin/TSH/Lyme titers/ischemic workup were all unrevealing). Contradictory to the criteria, our patient did not have any histological diagnosis of extra-cardiac sarcoidosis. Thus, a definite diagnosis of cardiac sarcoidosis remains challenging (4). Finally, current treatment seems to favor immunosuppressive therapy and using imaging modalities in the monitoring of disease activity and response to therapy (6). Our patient was started on Methotrexate 15 mg weekly sub Q and Prednisone 40 mg daily with plans for outpatient follow up 6 weeks for Prednisone taper.
Conclusions: cardiac only sarcoidosis should be on differential for heart block.