Background: Patients (pts) with congestive heart failure (CHF) are at an increased risk for community-acquired bacterial pneumonia (CABP) [1]. Moreover, CABP itself and macrolide antibiotics, which current guidelines recommend for CABP treatment [2], are both associated with increased risk of cardiac events, including worsening heart failure [3,4]. Lefamulin (LEF) is a first-in-class systemic pleuromutilin antibiotic approved in the United States to treat adults with CABP [5]. We report pooled efficacy/safety outcomes in pts with history of CHF from the LEAP 1 and LEAP 2 phase 3 trials.
Methods: In LEAP 1, adults with CABP (Pneumonia Outcomes Research Team [PORT] risk class III–V) received intravenous (IV) LEF 150 mg every 12 hours (q12h) for 5–7 days or moxifloxacin (MOX) 400 mg every 24 hours (q24h) for 7 days, with optional IV-to-oral switch (600 mg LEF q12h or 400 mg MOX q24h) [6]. In LEAP 2, adults with CABP (PORT II–IV) received oral LEF 600 mg q12h for 5 days or MOX 400 mg q24h for 7 days [7]. Both studies assessed early clinical response (ECR) at 96±24 hours after first study drug dose in the intent-to-treat (ITT; all randomized pts) population (US Food and Drug Administration primary endpoint) and investigator assessment of clinical response (IACR) at test of cure (TOC; 5–10 days after last study drug dose) in the modified ITT (received ≥1 dose of study drug) and clinically evaluable (met predefined evaluability criteria) populations (European Medicines Agency coprimary endpoints). Pooled analyses used a 10% noninferiority margin. The study protocol was approved by an independent ethics committee or institutional review board at each site, and each pt (or legally authorized representative) provided written informed consent.
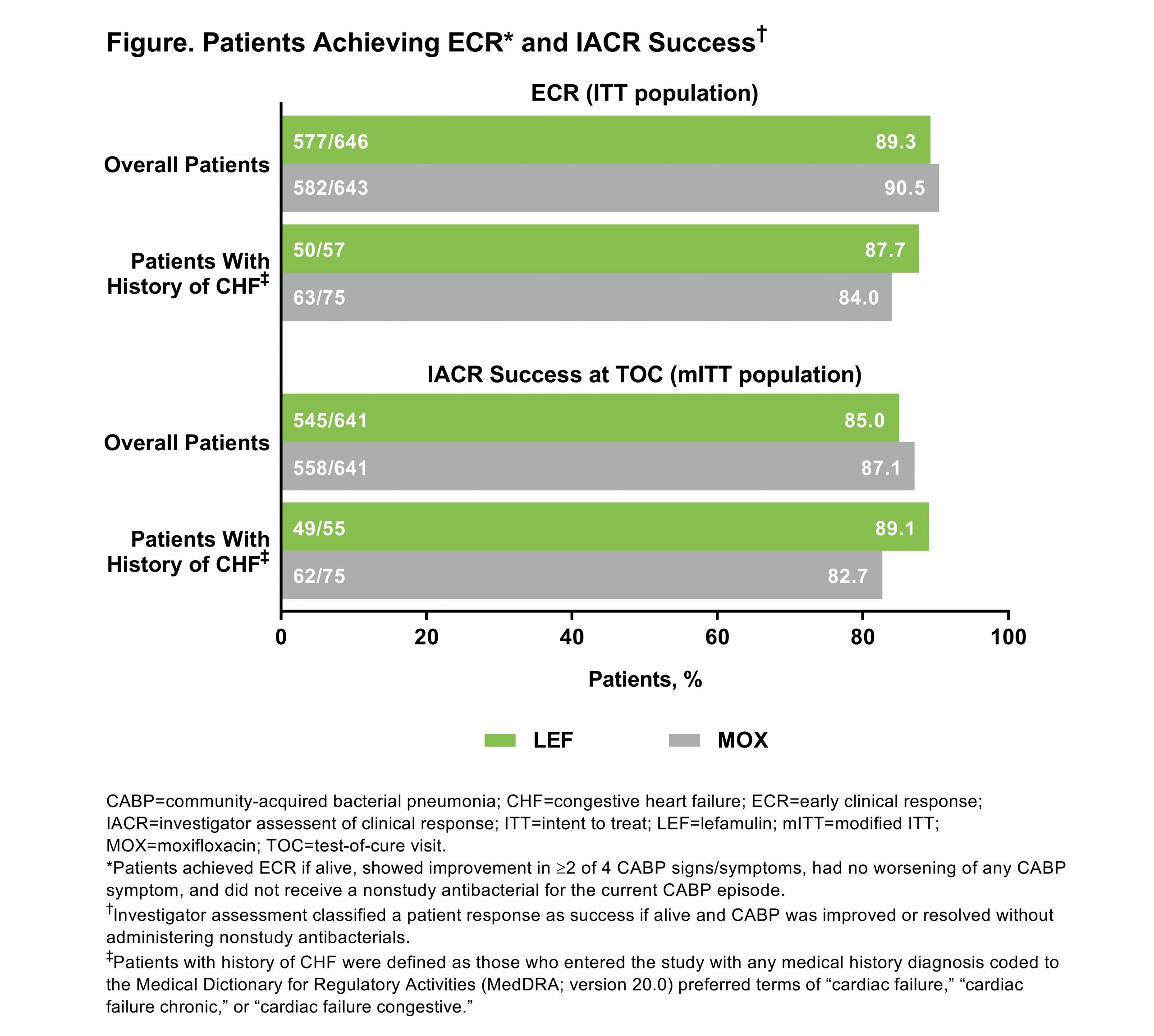
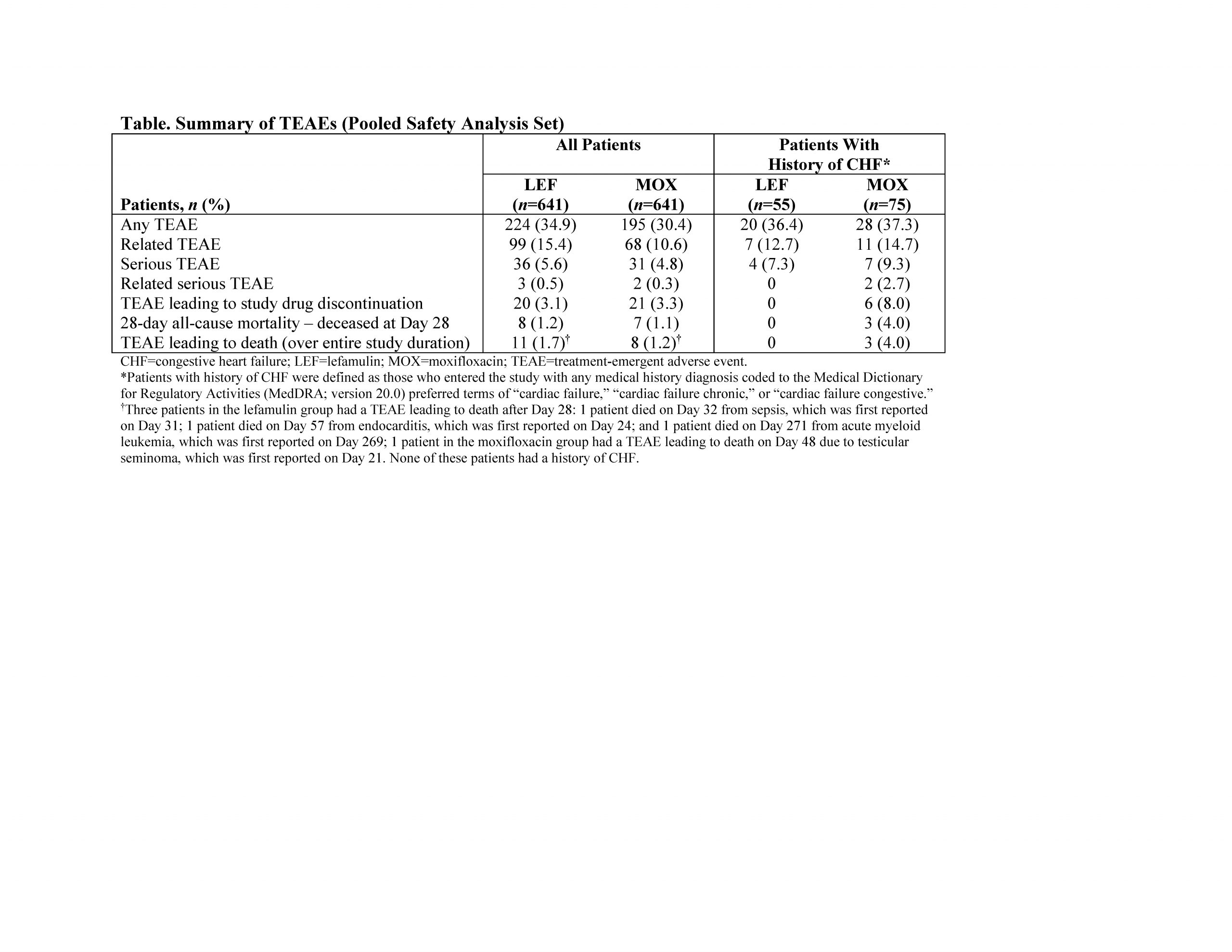
Results: In the pooled ITT population, 1289 pts were randomized to LEF (n=646) or MOX (n=643). Compared with the overall population, pts with history of CHF (LEF 8.8% [57/646], MOX 11.7% [75/643]) were older (median [range] age: overall 61 [19–97] years, CHF 71 [45–91] years) and more likely to have a severe PORT risk classification (PORT risk class IV/V: overall 18.5% [238/1289], CHF 40.9% [54/132]) and history of hypertension (overall 38.9% [n=501], CHF 68.9% [n=91]). ECR rates and IACR success rates were high and similar overall and in the CHF group (Figure).Treatment-emergent adverse event (TEAE) rates were similar in both groups (Table). The most common TEAE in the CHF group was diarrhea (LEF 3.6% [2/55], MOX 4.0% [3/75]), consistent with overall study results (LEF 7.3% [47/641], MOX 3.9% [25/641]). In the overall population, 2.8% of pts experienced TEAEs in the cardiac disorder system organ class (LEF 2.5% [n=16], MOX 3.1% [n=20]); in the CHF group, 5.4% of pts experienced cardiac disorder TEAEs (LEF 3.6% [n=2], MOX 6.7% [n=5]). The TEAE of prolonged QT interval was observed in 0.6% [n=4] and 0.8% [n=5] with LEF and MOX, respectively, in the overall group and 1.8% [n=1] and 4.0% [n=3] in the CHF group. Among pts with history of CHF, no pts treated with LEF discontinued study drug due to TEAEs or died (Table); 8.0% (n=6) of pts treated with MOX discontinued study drug due to TEAEs and 3 died (1 each due to cerebrovascular accident [Day 4], hematemesis/hemorrhagic shock [Day 1], and cardiac arrest [Day 18]).
Conclusions: Pooled data from these 2 pivotal phase 3 trials of pts with CABP showed high efficacy and good safety and tolerability for LEF. These data suggest that pts with history of CHF can be managed effectively and safely with IV or oral LEF as an alternative to fluoroquinolones for the treatment of CABP.